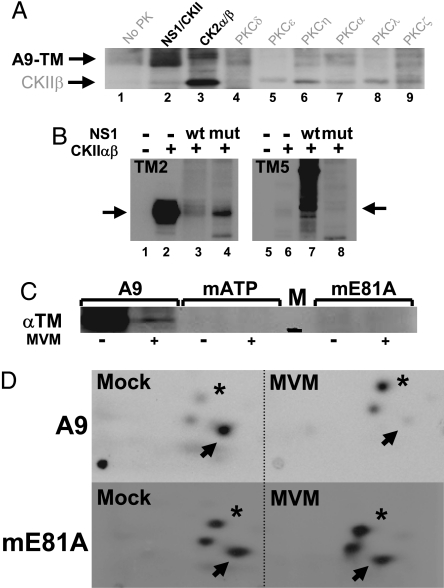
Fig. 2.
In vitro and in vivo phosphorylation of A9 cell tropomyosin. (A and B) In vitro kinase assays of the indicated PK substrate combinations were performed in the presence of [γ-32P]ATP and analyzed by SDS/PAGE and autoradiography. (A) A9-TM purified by NS1 affinity (Fig. 1B lane 4), alone (no PK), or combined with similarly purified NS1–CKIIα (Fig. 1B lane 3) or with the indicated recombinant PK. Migrations of TM (A9-TM) and recombinant CKIIβ are indicated. (B) Phosphorylation of recombinant TM2 (Left) and TM5 (Right) without (lanes 1 and 5) or with CKIIαβ in the absence (lanes 2 and 6) or presence of WT (lanes 3 and 7) or mutant S473A (lanes 4 and 8) GST-NS1. The migration of monomeric TM is indicated by arrows. (C and D) MVM infection and functional CKIIα were tested for their impact on the in vivo phosphorylation of TM. A9 cells or derivatives expressing the dominant-negative CKII(mATP) or CKII(mE81A) were infected (or not) with MVM (30 pfu per cell) and subjected to metabolic 32P labeling. TM was isolated by immunoprecipation and analyzed by SDS/PAGE (C), with reference to a 30-kDa marker (black bar in lane M). Bands corresponding to phospholabeled TM were excised, and their tryptic phosphopeptide patterns were analyzed (D). Phosphopeptides characteristically overrepresented in mock- (arrow) or MVM (asterisk)-infected cells are marked.