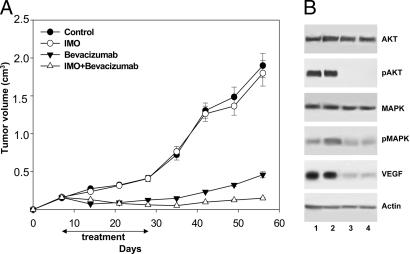
Fig. 4.
Effect of the combination of IMO with bevacizumab in mice bearing cetuximab-resistant GEO-CR tumor xenografts. (A) Seven days after GEO-CR tumor injection, mice were randomized (10 per group) to receive i.p. IMO, 1 mg/kg three times per week for 4 weeks; i.p. bevacizumab, 5 mg/kg, twice per week for 3 weeks, or the combination of these agents, on days 7–11, 14–18, and 21–25, continuing only IMO on days 28–32. Inhibition of growth was significantly different in the IMO plus bevacizumab-treated group vs. the control group, the IMO alone group, and the bevacizumab alone group (two-sided P < 0.0001 for each comparison). (B) Western blot analysis was performed on total lysates from tumor specimens of two mice killed on day 25 and treated as in Fig. 2. Lane 1, untreated control; lane 2, IMO; lane 3, bevacizumab; and lane 4, IMO plus bevacizumab. Doses and time of administration are indicated in Materials and Methods. Error bars indicate SD.