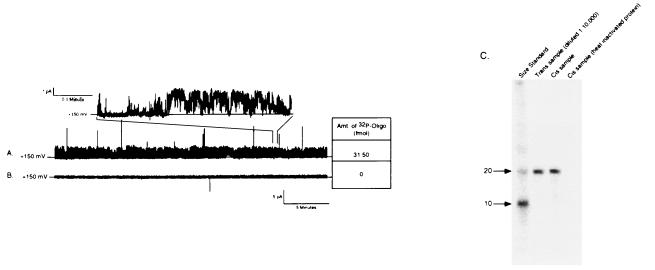
Figure 5.
Direct measurement of oligonucleotide movement across the lipid bilayer. Movement of oligonucleotide across the bilayer was confirmed by tracking the movement of [32P]S-Oligo. [32P]S-Oligo was added to the trans buffer chamber (input chamber) and 20–40 min later samples were collect from both buffer chambers. Radioactivity in the samples was measured by liquid scintillation and analyzed by gel electrophoresis. (A) Representative 30-min current trace obtained at a holding potential of +150 mV. The channel was highly active with clear transition between open and closed states (Inset). During the 30-min collection period approximately 32 fmol of [32P]S-Oligo moved across the lipid membrane. (B) A 30-min current trace obtained from an experiment in which NAC was heat-denatured before forming PLs. Channel activity was not observed and [32P]S-Oligo was not detected in the collection chamber, indicating that oligonucleotide did not move across the bilayer when NAC was rendered inactive. (C) A representative autoradiogram from an experiment to confirm that full-length oligonucleotide moved across the membrane. Samples from the trans (input) and cis (collection) chambers were loaded on a 15% polyacrylamide/7 M urea gel. Before loading, radioactivity in the samples was measured and appropriately diluted to load approximately the same number of counts in each well. Lanes: 1, radiolabeled size markers; 2, sample from the input chamber (diluted 1:10,000 with deionized H2O); 3, sample from collection chamber after 35 min of channel activity; 4, sample from collection chamber obtained 1 h after adding heat-inactivated NAC. In the presence of active NAC, a single band comigrated with the 20-base size marker and the input chamber aliquot. There was no degradation of oligonucleotide as it crossed the bilayer and there was no free 32P detected in the collection chamber solution. Identical results were observed when experiments were performed with radiolabeled Oligo (data not shown).