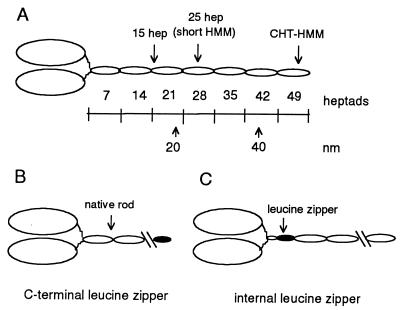
Figure 1.
(A) Schematic diagram of double-headed heavy meromyosin. An invariant proline (amino acid 849 in the gizzard heavy chain) marks the end of the head and the beginning of the heptad repeat in the rod. Each oval-shaped segment of the rod represents 7 heptad repeats. One heptad repeat has a repeated pattern of seven amino acids (abcdefg), where the a and d positions have a preponderance of hydrophobic residues (11). The rise per residue is 0.15 nm. CHT-HMM, HMM prepared by chymotryptic digestion of myosin. (B) Illustration of the C-terminal leucine zipper constructs. The C-terminal leucine zipper constructs are named for the number of heptads of native sequence before the leucine zipper sequence, e.g., 2-heptad/zipper has 2 heptads of rod sequence followed by the 32-aa leucine zipper sequence. (C) Illustration of the internal leucine zipper constructs, in which native rod sequence follows the leucine zipper sequence.