Abstract
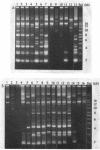
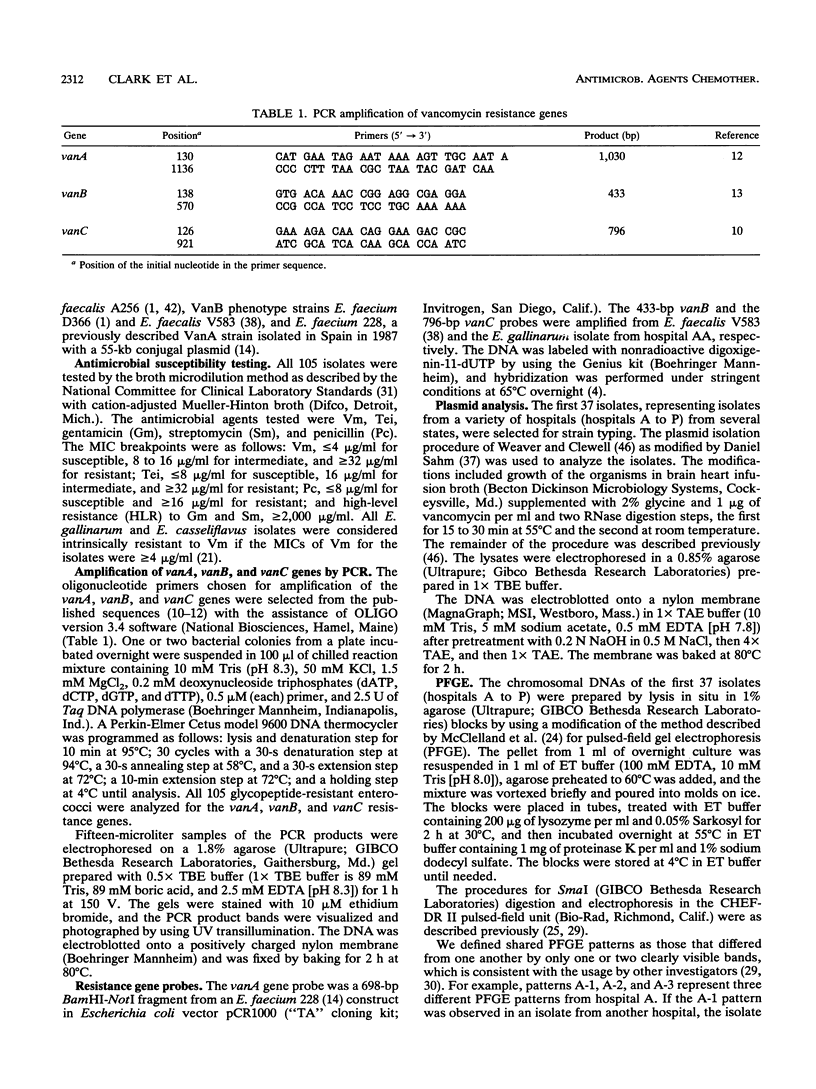
We examined 105 clinical isolates of glycopeptide-resistant enterococci collected from 31 U.S. hospitals in 14 states during May 1988 to July 1992. The isolates included 82 Enterococcus faecium, 8 E. faecalis, 6 Enterococcus spp., 5 E. gallinarum, 3 E. casseliflavus, and 1 E. raffinosus. The isolates were categorized into the following four phenotypes of glycopeptide resistance on the basis of their MIC patterns: (i) 70 VanA (vancomycin [Vm] MIC, > or = 64 micrograms/ml; teicoplanin [Tei] MIC, 16 to > or = 128 micrograms/ml), (ii) 26 VanB (Vm MIC, 16 to 1,024 micrograms/ml; Tei MIC, < or = 2 micrograms/ml), (iii) 5 VanC (Vm MIC, 4 to 16 micrograms/ml; Tei MIC, < or = 2 micrograms/ml) in E. gallinarum, and (iv) 3 E. casseliflavus and 1 E. raffinosus isolates for which Vm MICs were 4 to 16 micrograms/ml and Tei MICs were < or = 1 micrograms/ml were called unclassified. Of the 101 isolates with the VanA, VanB, and VanC phenotypes, 99 were confirmed by production of a specific 1,030-, 433-, or 796-bp polymerase chain reaction product, respectively, and hybridization with the respective gene probe. The vanA gene was also detected in the E. raffinosus isolate for which the Vm MIC was 16 micrograms/ml and the Tei MIC was 1 microgram/ml. The vanA gene was located on either a 34- or a 60-kb plasmid in all of the U.S. isolates examined. Pulsed-field gel electrophoresis demonstrated both intrahospital and interhospital diversity among Vmr enterococci in the United States and was more useful than plasmid analysis for epidemiologic studies.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur M., Molinas C., Courvalin P. The VanS-VanR two-component regulatory system controls synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J Bacteriol. 1992 Apr;174(8):2582–2591. doi: 10.1128/jb.174.8.2582-2591.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingen E. H., Denamur E., Lambert-Zechovsky N. Y., Elion J. Evidence for the genetic unrelatedness of nosocomial vancomycin-resistant Enterococcus faecium strains in a pediatric hospital. J Clin Microbiol. 1991 Sep;29(9):1888–1892. doi: 10.1128/jcm.29.9.1888-1892.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson-Noël A., Dutka-Malen S., Molinas C., Leclercq R., Courvalin P. Cloning and heterospecific expression of the resistance determinant vanA encoding high-level resistance to glycopeptides in Enterococcus faecium BM4147. Antimicrob Agents Chemother. 1990 May;34(5):924–927. doi: 10.1128/aac.34.5.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P. Genotypic approach to the study of bacterial resistance to antibiotics. Antimicrob Agents Chemother. 1991 Jun;35(6):1019–1023. doi: 10.1128/aac.35.6.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutka-Malen S., Leclercq R., Coutant V., Duval J., Courvalin P. Phenotypic and genotypic heterogeneity of glycopeptide resistance determinants in gram-positive bacteria. Antimicrob Agents Chemother. 1990 Oct;34(10):1875–1879. doi: 10.1128/aac.34.10.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutka-Malen S., Molinas C., Arthur M., Courvalin P. Sequence of the vanC gene of Enterococcus gallinarum BM4174 encoding a D-alanine:D-alanine ligase-related protein necessary for vancomycin resistance. Gene. 1992 Mar 1;112(1):53–58. doi: 10.1016/0378-1119(92)90302-6. [DOI] [PubMed] [Google Scholar]
- Dutka-Malen S., Molinas C., Arthur M., Courvalin P. The VANA glycopeptide resistance protein is related to D-alanyl-D-alanine ligase cell wall biosynthesis enzymes. Mol Gen Genet. 1990 Dec;224(3):364–372. doi: 10.1007/BF00262430. [DOI] [PubMed] [Google Scholar]
- Evers S., Sahm D. F., Courvalin P. The vanB gene of vancomycin-resistant Enterococcus faecalis V583 is structurally related to genes encoding D-Ala:D-Ala ligases and glycopeptide-resistance proteins VanA and VanC. Gene. 1993 Feb 14;124(1):143–144. doi: 10.1016/0378-1119(93)90779-3. [DOI] [PubMed] [Google Scholar]
- Handwerger S., Perlman D. C., Altarac D., McAuliffe V. Concomitant high-level vancomycin and penicillin resistance in clinical isolates of enterococci. Clin Infect Dis. 1992 Mar;14(3):655–661. doi: 10.1093/clinids/14.3.655. [DOI] [PubMed] [Google Scholar]
- Handwerger S., Pucci M. J., Kolokathis A. Vancomycin resistance is encoded on a pheromone response plasmid in Enterococcus faecium 228. Antimicrob Agents Chemother. 1990 Feb;34(2):358–360. doi: 10.1128/aac.34.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden M. K., Trenholme G. M., Schultz J. E., Sahm D. F. In vivo development of teicoplanin resistance in a VanB Enterococcus faecium isolate. J Infect Dis. 1993 May;167(5):1224–1227. doi: 10.1093/infdis/167.5.1224. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A., Archer G. L. New mechanisms of bacterial resistance to antimicrobial agents. N Engl J Med. 1991 Feb 28;324(9):601–612. doi: 10.1056/NEJM199102283240906. [DOI] [PubMed] [Google Scholar]
- Kaplan A. H., Gilligan P. H., Facklam R. R. Recovery of resistant enterococci during vancomycin prophylaxis. J Clin Microbiol. 1988 Jun;26(6):1216–1218. doi: 10.1128/jcm.26.6.1216-1218.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanfil L. V., Murphy M., Josephson A., Gaynes R., Mandel L., Hill B. C., Swenson J. M. A cluster of vancomycin-resistant Enterococcus faecium in an intensive care unit. Infect Control Hosp Epidemiol. 1992 Apr;13(4):195–200. doi: 10.1086/646509. [DOI] [PubMed] [Google Scholar]
- Leclercq R., Derlot E., Weber M., Duval J., Courvalin P. Transferable vancomycin and teicoplanin resistance in Enterococcus faecium. Antimicrob Agents Chemother. 1989 Jan;33(1):10–15. doi: 10.1128/aac.33.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq R., Dutka-Malen S., Brisson-Noël A., Molinas C., Derlot E., Arthur M., Duval J., Courvalin P. Resistance of enterococci to aminoglycosides and glycopeptides. Clin Infect Dis. 1992 Sep;15(3):495–501. doi: 10.1093/clind/15.3.495. [DOI] [PubMed] [Google Scholar]
- Leclercq R., Dutka-Malen S., Duval J., Courvalin P. Vancomycin resistance gene vanC is specific to Enterococcus gallinarum. Antimicrob Agents Chemother. 1992 Sep;36(9):2005–2008. doi: 10.1128/aac.36.9.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livornese L. L., Jr, Dias S., Samel C., Romanowski B., Taylor S., May P., Pitsakis P., Woods G., Kaye D., Levison M. E. Hospital-acquired infection with vancomycin-resistant Enterococcus faecium transmitted by electronic thermometers. Ann Intern Med. 1992 Jul 15;117(2):112–116. doi: 10.7326/0003-4819-117-2-112. [DOI] [PubMed] [Google Scholar]
- Markowitz S. M., Wells V. D., Williams D. S., Stuart C. G., Coudron P. E., Wong E. S. Antimicrobial susceptibility and molecular epidemiology of beta-lactamase-producing, aminoglycoside-resistant isolates of Enterococcus faecalis. Antimicrob Agents Chemother. 1991 Jun;35(6):1075–1080. doi: 10.1128/aac.35.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland M., Jones R., Patel Y., Nelson M. Restriction endonucleases for pulsed field mapping of bacterial genomes. Nucleic Acids Res. 1987 Aug 11;15(15):5985–6005. doi: 10.1093/nar/15.15.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda A. G., Singh K. V., Murray B. E. DNA fingerprinting of Enterococcus faecium by pulsed-field gel electrophoresis may be a useful epidemiologic tool. J Clin Microbiol. 1991 Dec;29(12):2752–2757. doi: 10.1128/jcm.29.12.2752-2757.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moellering R. C., Jr Emergence of Enterococcus as a significant pathogen. Clin Infect Dis. 1992 Jun;14(6):1173–1176. doi: 10.1093/clinids/14.6.1173. [DOI] [PubMed] [Google Scholar]
- Murray B. E., Lopardo H. A., Rubeglio E. A., Frosolono M., Singh K. V. Intrahospital spread of a single gentamicin-resistant, beta-lactamase-producing strain of Enterococcus faecalis in Argentina. Antimicrob Agents Chemother. 1992 Jan;36(1):230–232. doi: 10.1128/aac.36.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E. New aspects of antimicrobial resistance and the resulting therapeutic dilemmas. J Infect Dis. 1991 Jun;163(6):1184–1194. [PubMed] [Google Scholar]
- Murray B. E., Singh K. V., Heath J. D., Sharma B. R., Weinstock G. M. Comparison of genomic DNAs of different enterococcal isolates using restriction endonucleases with infrequent recognition sites. J Clin Microbiol. 1990 Sep;28(9):2059–2063. doi: 10.1128/jcm.28.9.2059-2063.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Singh K. V., Markowitz S. M., Lopardo H. A., Patterson J. E., Zervos M. J., Rubeglio E., Eliopoulos G. M., Rice L. B., Goldstein F. W. Evidence for clonal spread of a single strain of beta-lactamase-producing Enterococcus (Streptococcus) faecalis to six hospitals in five states. J Infect Dis. 1991 Apr;163(4):780–785. doi: 10.1093/infdis/163.4.780. [DOI] [PubMed] [Google Scholar]
- Patterson J. E., Masecar B. L., Kauffman C. A., Schaberg D. R., Hierholzer W. J., Jr, Zervos M. J. Gentamicin resistance plasmids of enterococci from diverse geographic areas are heterogeneous. J Infect Dis. 1988 Jul;158(1):212–216. doi: 10.1093/infdis/158.1.212. [DOI] [PubMed] [Google Scholar]
- Patterson J. E., Wanger A., Zscheck K. K., Zervos M. J., Murray B. E. Molecular epidemiology of beta-lactamase-producing enterococci. Antimicrob Agents Chemother. 1990 Feb;34(2):302–305. doi: 10.1128/aac.34.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintiliani R., Jr, Evers S., Courvalin P. The vanB gene confers various levels of self-transferable resistance to vancomycin in enterococci. J Infect Dis. 1993 May;167(5):1220–1223. doi: 10.1093/infdis/167.5.1220. [DOI] [PubMed] [Google Scholar]
- Rhinehart E., Smith N. E., Wennersten C., Gorss E., Freeman J., Eliopoulos G. M., Moellering R. C., Jr, Goldmann D. A. Rapid dissemination of beta-lactamase-producing, aminoglycoside-resistant Enterococcus faecalis among patients and staff on an infant-toddler surgical ward. N Engl J Med. 1990 Dec 27;323(26):1814–1818. doi: 10.1056/NEJM199012273232606. [DOI] [PubMed] [Google Scholar]
- Rice L. B., Eliopoulos G. M., Wennersten C., Goldmann D., Jacoby G. A., Moellering R. C., Jr Chromosomally mediated beta-lactamase production and gentamicin resistance in Enterococcus faecalis. Antimicrob Agents Chemother. 1991 Feb;35(2):272–276. doi: 10.1128/aac.35.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahm D. F., Kissinger J., Gilmore M. S., Murray P. R., Mulder R., Solliday J., Clarke B. In vitro susceptibility studies of vancomycin-resistant Enterococcus faecalis. Antimicrob Agents Chemother. 1989 Sep;33(9):1588–1591. doi: 10.1128/aac.33.9.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahm D. F., Olsen L. In vitro detection of enterococcal vancomycin resistance. Antimicrob Agents Chemother. 1990 Sep;34(9):1846–1848. doi: 10.1128/aac.34.9.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaberg D. R., Culver D. H., Gaynes R. P. Major trends in the microbial etiology of nosocomial infection. Am J Med. 1991 Sep 16;91(3B):72S–75S. doi: 10.1016/0002-9343(91)90346-y. [DOI] [PubMed] [Google Scholar]
- Shlaes D. M., Binczewski B. Enterococcal resistance to vancomycin and related cyclic glycopeptide antibiotics. Eur J Clin Microbiol Infect Dis. 1990 Feb;9(2):106–110. doi: 10.1007/BF01963634. [DOI] [PubMed] [Google Scholar]
- Shlaes D. M., Bouvet A., Devine C., Shlaes J. H., al-Obeid S., Williamson R. Inducible, transferable resistance to vancomycin in Enterococcus faecalis A256. Antimicrob Agents Chemother. 1989 Feb;33(2):198–203. doi: 10.1128/aac.33.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson J. M., Ferraro M. J., Sahm D. F., Charache P., Tenover F. C. New vancomycin disk diffusion breakpoints for enterococci. The National Committee for Clinical Laboratory Standards Working Group on Enterococci. J Clin Microbiol. 1992 Oct;30(10):2525–2528. doi: 10.1128/jcm.30.10.2525-2528.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover F. C., Tokars J., Swenson J., Paul S., Spitalny K., Jarvis W. Ability of clinical laboratories to detect antimicrobial agent-resistant enterococci. J Clin Microbiol. 1993 Jul;31(7):1695–1699. doi: 10.1128/jcm.31.7.1695-1699.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent S., Minkler P., Bincziewski B., Etter L., Shlaes D. M. Vancomycin resistance in Enterococcus gallinarum. Antimicrob Agents Chemother. 1992 Jul;36(7):1392–1399. doi: 10.1128/aac.36.7.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver K. E., Clewell D. B. Regulation of the pAD1 sex pheromone response in Enterococcus faecalis: construction and characterization of lacZ transcriptional fusions in a key control region of the plasmid. J Bacteriol. 1988 Sep;170(9):4343–4352. doi: 10.1128/jb.170.9.4343-4352.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells V. D., Wong E. S., Murray B. E., Coudron P. E., Williams D. S., Markowitz S. M. Infections due to beta-lactamase-producing, high-level gentamicin-resistant Enterococcus faecalis. Ann Intern Med. 1992 Feb 15;116(4):285–292. doi: 10.7326/0003-4819-116-4-285. [DOI] [PubMed] [Google Scholar]
- Willey B. M., Kreiswirth B. N., Simor A. E., Willaims G., Scriver S. R., Phillips A., Low D. E. Detection of vancomycin resistance in Enterococcus species. J Clin Microbiol. 1992 Jul;30(7):1621–1624. doi: 10.1128/jcm.30.7.1621-1624.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zervos M. J., Kauffman C. A., Therasse P. M., Bergman A. G., Mikesell T. S., Schaberg D. R. Nosocomial infection by gentamicin-resistant Streptococcus faecalis. An epidemiologic study. Ann Intern Med. 1987 May;106(5):687–691. doi: 10.7326/0003-4819-106-5-687. [DOI] [PubMed] [Google Scholar]
- al-Obeid S., Collatz E., Gutmann L. Mechanism of resistance to vancomycin in Enterococcus faecium D366 and Enterococcus faecalis A256. Antimicrob Agents Chemother. 1990 Feb;34(2):252–256. doi: 10.1128/aac.34.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]