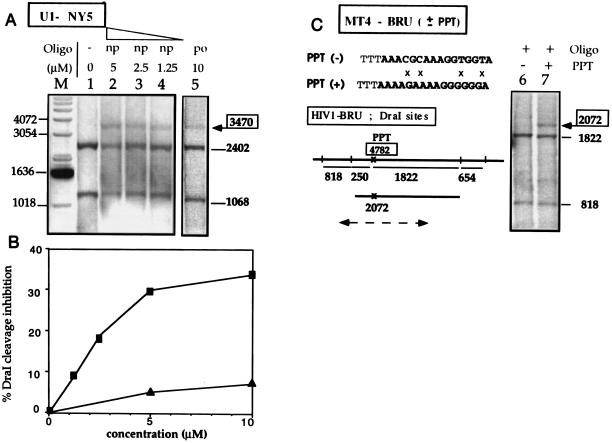
Figure 2.
Triplex formation in nuclei of permeabilized cells. Chronically infected cells were permeabilized with digitonin, incubated in the triplex-forming buffer in the absence or in the presence of Pso-15TCG, and irradiated. After cell lysis, followed by proteinase K and RNase A treatments, the genomic DNA was prepared and analyzed by DraI protection assay. Chronically infected cells were used (U1–NY5, MT4–BRU). They were obtained by infection with different HIV-1 strains (called NY5 and BRU) and differed from each other by the HIV-1 sequence. However the PPT triplex site sequence was the same in all strains except in the MT4–BRU(−) cell line where the PPT sequence was mutated at four positions (see C). These sequence differences led to slightly different DraI cleavage patterns, as indicated under the gels, but even though the fragment lengths were modified from one cell line to the other, specific triplex formation was always correlated with the appearance of a longer fragment of well-defined size; probe location is shown by a broken line for MT4-BRU (see Fig. 1 for U1-NY5). (A) U1 cells (HIV-1–NY5 sequence); the po or np oligomers and the concentration of the Pso-15TCG are shown by the gels. (B) Quantitation of DraI cleavage inhibition at the PPT triplex site in nuclei of permeabilized U1 cells versus oligonucleotide concentration (np, ▪; po, ▴). (C) MT4 cells [HIV-1–BRU(+), lane 7; or HIV-1-BRU(−), lane 6] treated with Pso-15TCG(po). The sequence of the mutated PPT [PPT(−)] is indicated and mismatches with the wild PPT [PPT(+)] are indicated by crosses (×).