Figure 1.
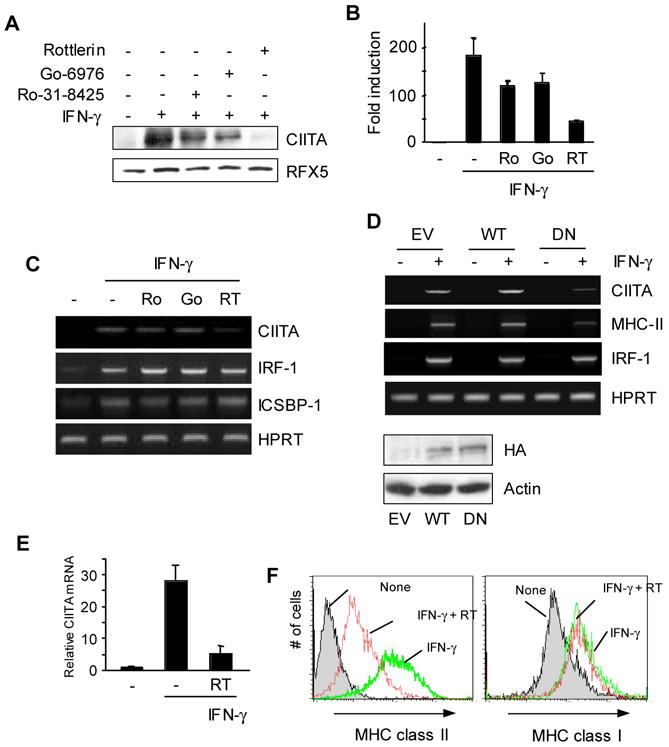
PKCδ regulates IFN-γ inducible expression of CIITA in macrophages. (A–C) RAW264.7 cells were pre-treated with each PKC inhibitor: Ro-31-8425 (500nM), Go-6976 (200nM), Rottlerin (5 μM), or DMSO for 30 min followed by IFN-γ for 8hrs. (A) Cell lysates were prepared to do immunoblot using the antibody as indicated. 50 μg of total lysates was loaded on a 6% gel. The same membrane was stripped and re-probed with an anti-RFX5 antibody. (B) RNA was prepared from RAW264.7 cells treated with PKC inhibitors as in (A). Type IV CIITA mRNA levels were determined by qRT-PCR. qRT-PCR data were normalized against mRNA levels of the GAPDH gene. Data are means ± SE of three independent experiments. (C) Other IFN-γ-inducible genes were not affected by PKCδ inhibition. RAW264.7 cells were treated with PKC inhibitors as in (A). mRNA was analyzed using RT-PCR for each gene as indicated. (D) DN PKCδ expressing cells have reduced levels of CIITA and MHC class II mRNA. RAW264.7 cells that were stably transfected with the empty vector (EV), wild type (WT), or a dominant negative (DN) mutant form of PKCδ were treated with IFN-γ for 12hrs. Expression of transfected PKCδ was determined by immunoblotting with an anti-HA antibody. (E) BM macrophages from C57BL/6 mice were pretreated with Rottlerin (5μM) for 30 min, followed by IFN-γ stimulation for 6 hrs. Type IV CIITA mRNA levels were measured by qRT-PCR. The relative mRNA levels were normalized to the GAPDH gene. (F) BM macrophages were pretreated with Rottlerin for 30 min, followed by IFN-γ stimulation for 24 h. Cell surface MHC class II and class I expression was analyzed by flow cytometry. Data are representative (A, C, D, and F) or means ± SE (B and E) of at least two independent experiments.
