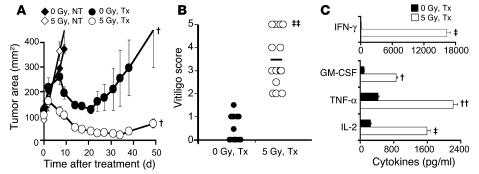
Figure 1. TBI enhances the function of adoptively transferred self/tumor-reactive pmel-1 T cells in mice genetically deficient in cytokine sinks and Tregs.
(A) TBI augmented antitumor responses in mice genetically deficient in cytokine sinks and Tregs. Rag2–/–γc–/– mice (deficient in T, B, and NK cells) bearing s.c. B16F10 tumors established for 10 days received nonmyeloablative 5 Gy TBI or were not irradiated (0 Gy). One day later, mice received an ACT treatment regimen consisting of the adoptive transfer of 105 cultured self/tumor reactive pmel-1 T cells, rFPhgp100 vaccination, and rhIL-2 or were left untreated (NT). Data (mean ± SEM; n = 5 per group) are representative of 4 independent experiments. (B) TBI enhanced autoimmune vitiligo in Rag2–/–γc–/– mice. Twenty-eight days after treatment, nonirradiated and irradiated Rag2–/–γc–/– mice were evaluated in a blinded fashion for the development of vitiligo. Each mouse was scored for degree of hypopigmentation on a scale of 0–5. Data (n = 14 per group) are representative of 2 independent experiments. Horizontal bars indicate means. (C) TBI enhanced the function of adoptively transferred pmel-1 CD8+ T cells. Five days after treatment, pmel-1–Thy1.1+ cells were isolated from spleens of irradiated and nonirradiated Rag2–/–γc–/– mice and were cocultured with irradiated splenocytes pulsed with 1 μM hgp10025–33. Secretion of IFN-γ, GM-CSF, TNF-α, and IL-2 in pmel-1 cells was analyzed. Unpulsed splenocytes were used as controls. Data (mean ± SEM; n = 3 per group) are representative of 2 independent experiments. †P = 0.05, ††P < 0.05, ‡P < 0.01, ‡‡P < 0.001 versus nonirradiated treated mice. Tx, treatment.