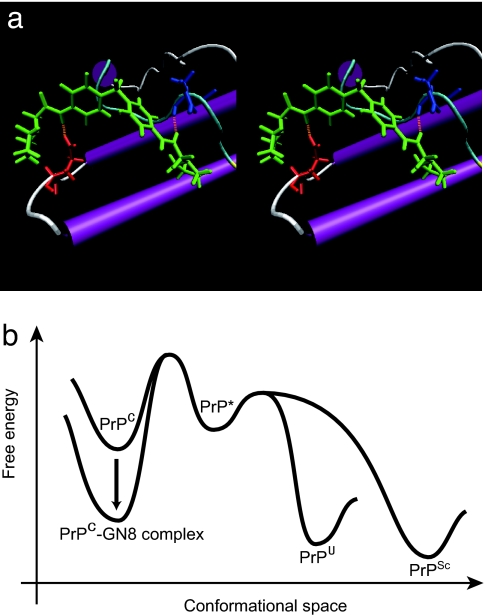
Fig. 3.
Inhibitory mechanism of GN8 for pathogenic conversion. (a) Stereoview of the optimized complex structure of the GN8 and mouse PrPC, with putative hydrogen bonds, calculated by using ICM version 3.0. Presumed hydrogen bonds between GN8 (green) and E196 (red) and between GN8 (green) and N159 (blue) are shown in orange (dotted lines). The images were created with VMD (52). (b) Illustration of the Gibbs free energy as a function of the conformational space to explain the inhibitory mechanism of a chemical chaperone, GN8. GN8 stabilizes the PrPC conformation and reduces the population of PrP*, PrPSc, and PrPU. PrP* may not interact with GN8, because the specific conformation around the binding sites would be lost (14). Thus, the free energy level of PrP*, PrPU, and PrPSc would not change much in the presence of GN8.