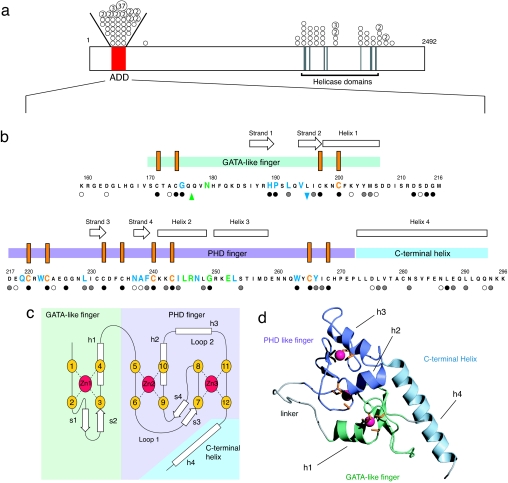
Fig. 1.
ATRX protein sequence, structure, and disease-associated mutations. (a) The locations of the highly conserved N-terminal cysteine-rich domain and the C-terminal helicase-like domain are shown. The positions of missense mutations are indicated with circles and the number of times (>1) the mutation has been identified in unrelated individuals is indicated within relevant circles. All of the circles drawn between the oblique lines above the bar refer to mutations within the ADD domain. (b) Locations of mutations and secondary structural elements in the ADD domain. The N-terminal GATA-like zinc finger is indicated by a light green bar, the PHD finger by a mauve bar, and the C-terminal extension by a light blue bar. The conserved cysteine residues are marked as orange vertical bars. Missense mutations are highlighted in green (surface), blue (buried), and orange (cysteines); the insertion mutation is highlighted by an upward green arrow and the deletion by a downward blue arrow. Residues where there is homology across the whole family of ADD domain sequences (ATRX, DNMT3A, DNMT3B, and DNMT3L) are marked with filled circles (absolute conservation), gray circles (strong conservation), and open circles (weak conservation); for the full alignment, see SI Fig. 5. (c) Schematic showing the zinc-binding topology and secondary structure elements of the ADD domain, color scheme as for b. β-Strands are labeled s1–s4 and helices h1–h4. The zinc binding within the PHD finger has the “cross-braced” topology characteristic of such domains, with each zinc coordinated by a noncontiguous set of ligands. (d) Ribbon representation of the NMR structure of the ADD domain (lowest energy structure from the accepted ensemble of 32) of ATRX. The GATA-like finger is shown in green, the PHD finger in mauve, and the C-terminal helix in blue. Linker and unstructured regions are shown in gray, zinc atoms in pink, and side chains of the zinc coordinating cysteines in orange.