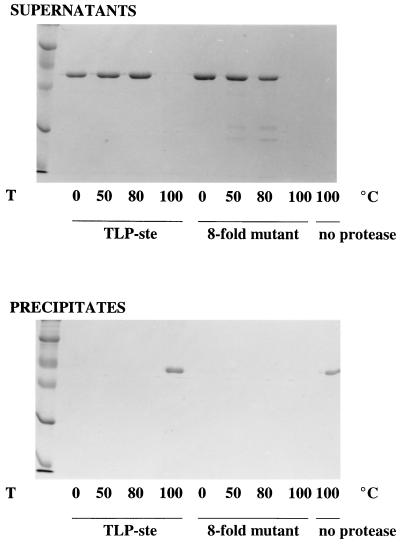
Figure 4.
Hydrolysis of protease-resistant α-amylase from Bacillus licheniformis by the 8-fold mutant. B. licheniformis α-amylase was incubated with purified TLP-ste, the 8-fold mutant, or without protease for 60 min at the temperature indicated. After incubation the samples were cooled on ice, which resulted in aggregation of the substrate in the samples that had been incubated at 100°C. Precipitates (observed only in the 100°C samples) were collected by centrifugation and redissolved in 6 M urea. Both supernatants (Upper) and redissolved precipitates (Lower) were subjected to standard SDS/PAGE. No significant degradation of α-amylase occurred at temperatures of 80°C and lower, irrespective of the enzyme used. In cases where the samples were incubated at 100°C without added protease or with TLP-ste the aggregate formed after cooling contained mature α-amylase (Lower), indicating that no hydrolysis had occurred. The B. licheniformis α-amylase that was incubated with the 8-fold mutant at 100°C was completely hydrolyzed, and no aggregate was formed.