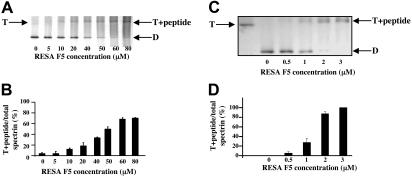
Figure 3.
Stabilization of spectrin tetramer by RESA F5 fragment. Panels A and B show the composition of spectrin extracted at 37°C from erythrocyte membranes previously resealed without or with the indicated concentrations of RESA F5. Spectrin components were separated by nondenaturing gel electrophoresis in the cold. The positions of migration of dimer (D), tetramer (T), and the tetramer-RESA-F5 complex (T + peptide) are indicated. Panels C and D show suppression of conversion of spectrin tetramer to dimer by RESA F5 in solution. Spectrin tetramer in the low-ionic-strength extraction medium was incubated with the RESA fragment at the indicated concentrations at 37°C for 1 hour, followed by gel electrophoresis, as before. The best-fit of binding data in situ at 37°C shown in panel B corresponds to an association constant of approximately 2 × 104 M−1, while that of binding data shown in panel D results in an association constant of approximately 1 × 106 M−1. (B,D) Results represent the mean value of triplicate samples.