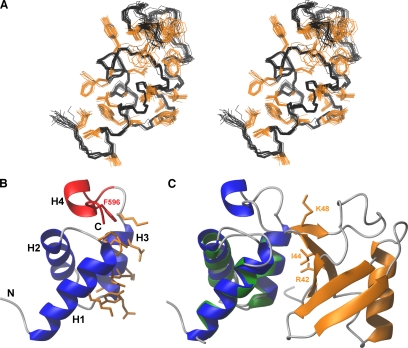
Figure 1.
NMR structure of the Mex67 UBA domain and a model of its likely interaction interface with ubiquitin. (A) Stereo view of the ensemble of 20 NMR structures having the lowest total energy. Positions of the backbone-heavy atoms are given in black, whereas those of hydrophobic and aromatic side chains are in orange. (B) Representation of the secondary structure elements of the UBA-Mex67 closest to the mean of the NMR ensemble. The residues of UBA-Mex67 that may interact with monoubiquitin are in orange. The region of the structure deleted in UBAΔH4-Mex67 and Phe596 are highlighted in red. (C) Prediction of the likely docking geometry of the Mex67 UBA domain onto ubiquitin by analogy with the structure of the Ede1:ubiquitin complex (PDB:2G3Q). Ubiquitin is orange, and the Ede1 UBA domain is green. Helices H1-H3 of the UBA-Mex67 domain (blue) were superimposed on the corresponding helices in the Ede1 structure. The side chains of the key residues Lys48, Ile44, and Arg42 on ubiquitin are also shown in orange.