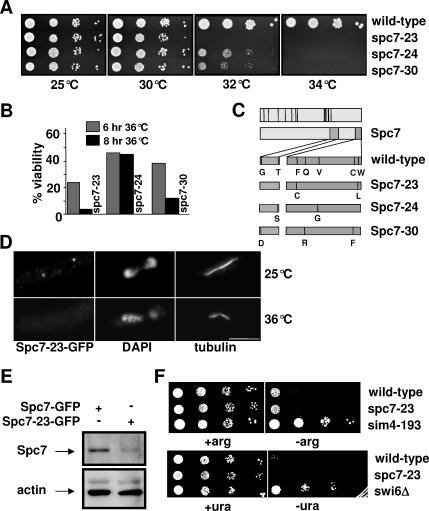
Figure 1.
Spc7 is not involved in centromere silencing. Serial dilution patch tests (104 to 101 cells) of wild-type, spc7-23, spc7-24, and spc7-30 strains grown at the indicated temperatures for 3–4 d. (B) Viability of wild-type and spc7 mutant strains incubated at 36°C for 6 or 8 h. (C) Top diagram, black bars indicate conserved Spc7 residues; bottom diagram, amino acid changes found in the Spc7 mutant proteins in comparison to wild-type Spc7. (D) Kinetochore localization of Spc7-23. Cells expressing the mutant Spc7-23-GFP protein were incubated at 25°C (top lane) or at 36°C for 6 h (bottom lane), fixed, and stained with DAPI, anti-tubulin antibody, and anti-GFP-antibody. Bar, 5 μm. (E) Endogenous Spc7-GFP or Spc7-23-GFP protein isolated from cells grown asynchronously at 32°C for 6 h. Protein extracts prepared from these strains were used for immunoprecipitations using an anti-GFP antibody, followed by Western blotting using the same antibody. Protein extracts used had a similar protein concentration. Actin was used as a loading control. Spc7-23 protein is reduced relative to the wild-type protein by 70%. (F) Serial dilution patch tests (104 to 101 cells) of wild-type, spc7-23, and sim4-193 cells that have the promoter-crippled arg3+ gene inserted at cen1 (top panels) or ura4+ inserted at otr2 (wild-type, spc7-23, and swi6Δ cells). Cells were incubated on selective medium with (+arg) or without (−arg) arginine or with (+ura) and without (−ura) uracil at 25°C for 6 d.