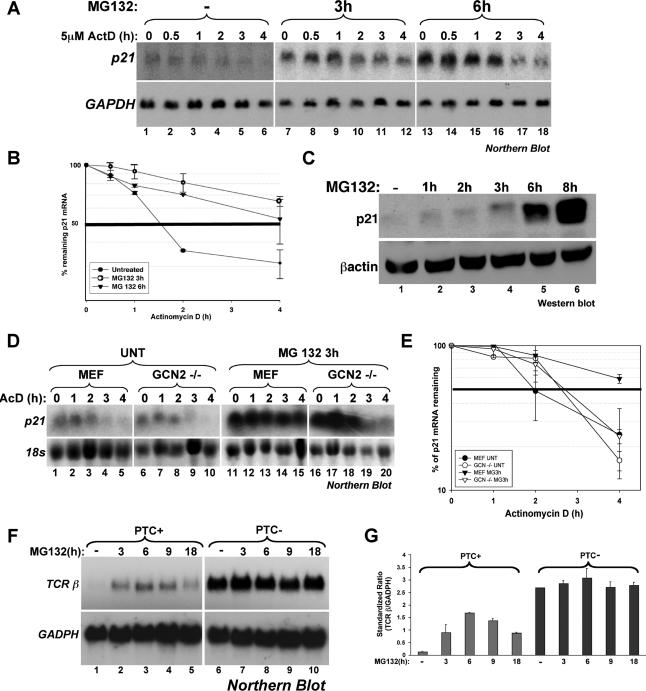
Figure 9.
The inhibition of the proteasome blocks both ARE-mediated decay and NMD. (A and B) Effect of MG132 treatment on the half-life of p21cip1 mRNA in HeLa cells. (A) HeLa cells were first treated with or without MG132 for 3 h and then incubated with 5 μg/ml actinomycin D (ActD) for the indicated times. Total RNA was prepared and the expression levels of p21cip1 mRNA was determined using Northern blot analysis. Endogenous GAPDH mRNA was used as a loading control. (B) The expression of p21cip1 mRNA was quantified using the ImageQuant software program. The expression levels of p21cip1 mRNA were then standardized against GAPDH message and plotted as the percentage of remaining mRNA compared with message levels at the 0 time point (where there is 100% maximum mRNA level). Error bars, SD of three independent experiments. (C) Effect of MG132-treatment on the synthesis of the p21cip1 protein in HeLa cells. Total cell extracts prepared from HeLa cells treated with 10 μM MG132 for the indicated time periods were harvested and used for Western blot analysis. The membrane was probed for p21cip1 and β-actin proteins. (D and E) The GCN2 kinase seems to be required for the MG132-mediated stabilization of the p21cip1 mRNA. (D) Wild-type MEFs and MEFs derived from GCN2−/− mice were treated as in A, and the expression levels of mouse p21cip1 mRNA was determined using Northern blot analysis. (E) The expression of p21cip1 mRNA was quantified as in A. Levels were standardized against 18S rRNA. Error bars, SD of two independent experiments. (F and G) UPS inhibition by MG132 interferes with the nonsense-mediated decay pathway. (F) HeLa cells stably expressing T-cell receptor beta minigene wild type (TCR-β-wt) or a TCR-β minigene containing a PTC were treated with 10 μM MG132 for different time points, and the levels of TCR-β mRNA were analyzed by Northern blot. (G) TCR-β mRNA levels were standardized as described in A and B. Error bars, SD of two independent experiments.