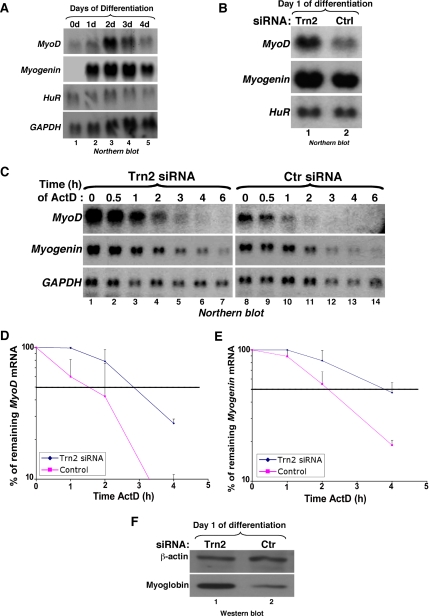
Figure 5.
TRN2 knockdown increases the steady state levels and stability of MyoD and myogenin mRNAs. (A) Northern blotting was performed on total mRNA that was isolated from C2C12 cells on each day of differentiation using radiolabeled probes against MyoD, Myogenin, HuR, and GAPDH messages. (B) Total RNA was isolated from TRN2 or control (Ctrl) siRNA-treated C2C12 cells, and Northern blotting was performed using specific 32P-labeled cDNA probes for MyoD, myogenin, and HuR messages to assess steady state levels of MyoD and myogenin mRNAs. (C) The stability of MyoD and myogenin mRNA was assessed in TRN2 siRNA- and control-treated cells by adding the transcriptional inhibitor actinomycin D (ActD) 16 h after differentiation induction and harvesting RNA at the indicated time points after ActD treatment for Northern blotting. GAPDH was used as a loading control. (D and E) The stability of the MyoD (D) and myogenin (E) mRNAs in cells treated with TRN2 and control siRNAs was determined and quantified from Northern blotting using the Image Quant computer program (Molecular Dynamics, Sunnyvale, CA) to measure band intensities. The percent remaining of both mRNAs was defined as the relative intensity of each message to GAPDH bands at each time point and was calculated as a percent of the abundance of each message at 0 h of ActD treatment and plotted on a logarithmic curve. The dotted horizontal line represents 50% remaining mRNA relative the original abundance of the message and therefore the half-life of the message under the different cell treatments. Error bars, SD of two independent experiments. (F) Total cell extracts were prepared from C2C12 cells at t = 16 h of differentiation (day 1), the same time point used for ActD treatment, and SDS-PAGE was performed for Western blot analysis using antibodies for myoglobin and β-actin.