Figure 5.
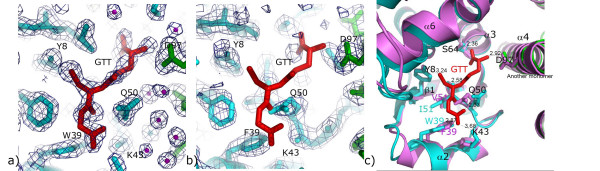
G-site features. a) G-site of Na-GST-2 shows unambiguous electron density for glutathione (GTT) in 2Fo-Fc maps contoured at 1 sigma. b) No such density is visible in the G-site of Na-GST-1. c) Alignment of G-sites of Na-GST-2 and Na-GST-1. GTT is modeled from Na-GST-2 structure. The monomers of Na-GST-2 dimer are colored in cyan and green, while Na-GST-1 is colored in violet. Trp39 forms a hydrogen bond with glutathione in Na-GST-2 which is replaced with Phe39 in Na-GST-1. Gln50 is conserved in both Na-GST-2 and Na-GST-1, but the side chain is flipped such that glutathione cannot fit in Na-GST-1 G-site. The catalytic Tyr8 maintains its conformation in both structures. Polar interactions and distances are also shown.
