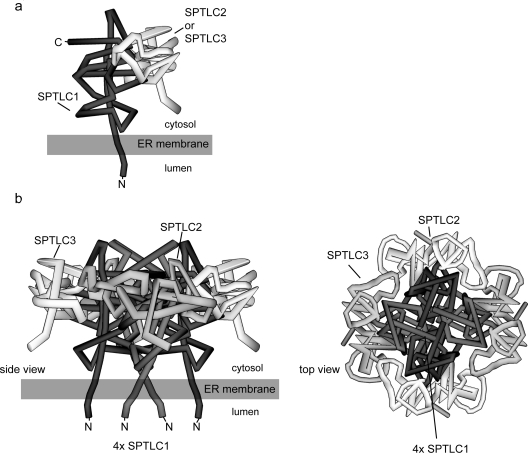
Figure 4. Theoretical model of the SPT complex structure.
The model is based on the following assumptions. First, that the N-terminal end of SPTLC1 is bound to the ER membrane; secondly, that SPTLC2 and SPTLC3 are not interacting directly with the ER membrane; thirdly, that the active site of SPT is formed at the interface between two monomers; and, finally, that SPTLC2 can be dynamically replaced by SPTLC3 within the complex. (a) The SPTLC2 or SPTLC3 monomers form a dimeric base structure with SPTLC1, which is then assembled to its final octameric state. (b) Putative model of the octameric SPT complex. In the conformation shown, two SPTLC1–SPTLC2 and two SPTLC1–SPTLC3 dimers are assembled together to form an octameric circular structure. The stoichiometry of SPTLC2 and SPTLC3 within the complex depends on their individual cellular expression levels. The final complex contains four active sites, which are located at the monomer–monomer interface. The N-termini of the SPTLC1 subunits are bound to the ER membrane in a manner that the catalytic head group protrudes towards the cytosolic compartment of the cell.