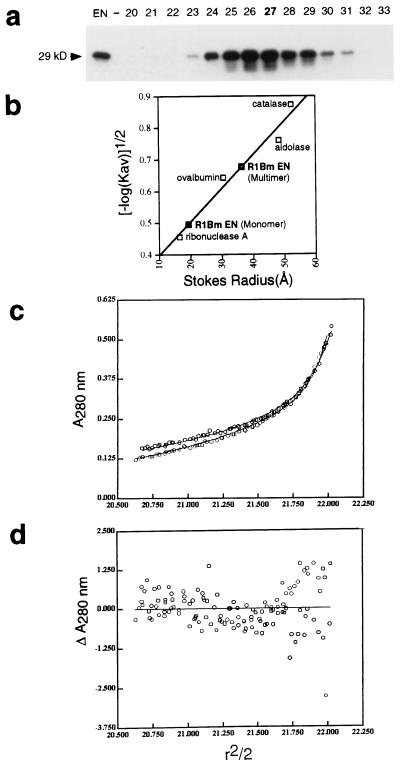
Figure 5.
R1Bm EN behaves as a multimer. (a) Purified R1Bm EN protein (24 μg) was applied to a sizing column with molecular weight standards, and the fractions were immunoblotted with the anti-tag antibody to detect EN protein. Nearly all of the EN protein (as well as bulk absorbance) peaked at fractions 23–31, but a second small peak was observed on a long exposure at fraction 42 (not shown) and is assumed to represent monomeric enzyme. (b) Determination of Stokes radius of R1Bm EN monomer and multimer by gel filtration chromatography at 4°C with the indicated standards. The EN monomer peak runs with a Stokes radius of 19 Å corresponding to a globular protein of 19 kDa even though the actual mass is 29 kDa, suggesting that the EN has weak affinity for the column matrix. The Stokes radius of the multimer (36 Å) corresponds to a globular protein of 81 kDa and thus is consistent with a spherical tetramer with weak affinity for the column matrix. (c and d) Sedimentation equilibrium data for R1Bm EN at 12,000 and 15,000 rpm at 4°C. c represents the actual data (open dots) and the results of a global fit of these two data sets to a monomer–tetramer–dodecamer model (lines; see Materials and Methods). d presents a composite residual plot for the global fit in c; r, radius. A random distribution of actual data points (dots) about the predicted value (line) indicates very good agreement with the model. The data rule out a pure monomeric state for the R1Bm EN. A monomer–tetramer–dodecamer model with a tetramer as the predominant species (92%) fit these data best. Thus both gel filtration and sedimentation equilibrium methods are consistent with a predominantly tetrameric enzyme.