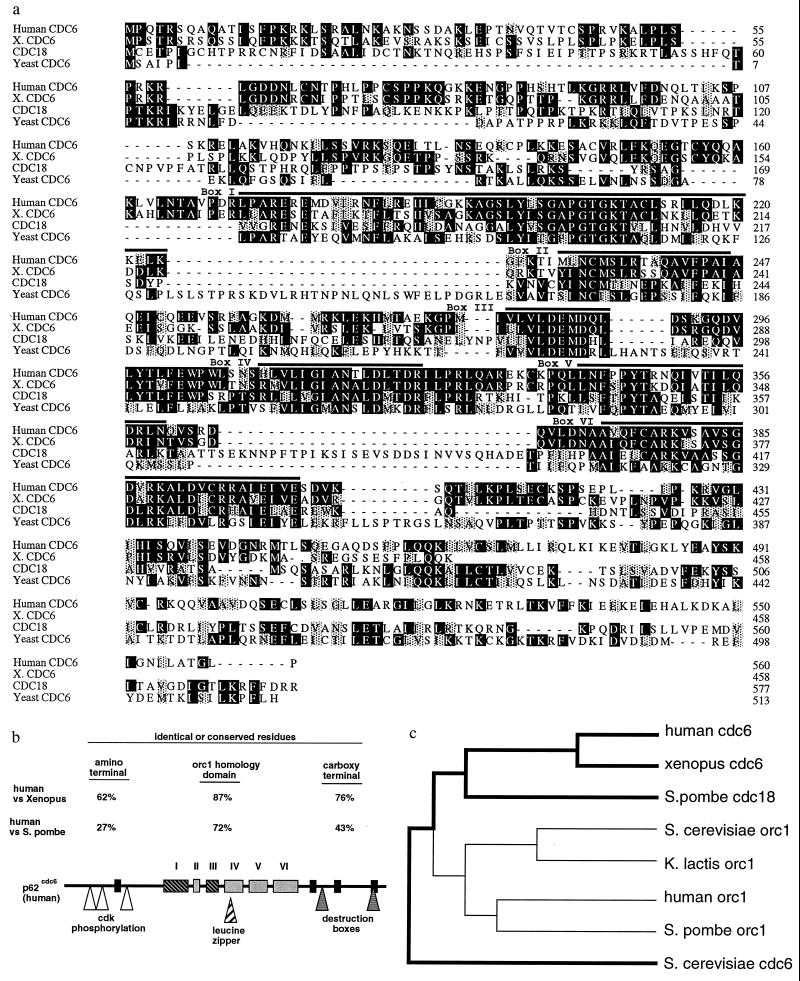
Figure 1.
(a) Multiple alignment of amino acid sequences of human p62cdc6 and related proteins from Xenopus laevis (partial sequence), Saccharomyces cerevisiae, and Schizosaccharomyces pombe. Amino acid residues that are identical are indicated by dark shading, and conservative substitutions are indicated by light shading. The six conserved sequence blocks (I–VI) previously found to be shared among the Orc1 family and fungal Cdc6p/cdc18+ proteins (4, 13), which include the nucleotide binding/ATPase domain (I and III), are found in the midportion of these proteins. Outside of this central domain, no regions of sequence similarity with Orc1-related proteins are evident. (b) Potential structural motifs and highly conserved regions of human p62cdc6. The regions similar to the Orc1 family of proteins are indicated (I–VI). Four other regions of near identity among the vertebrate and yeast Cdc6-related proteins (black rectangles) are present in the amino or carboxyl-terminal regions, and are not found in Orc1 proteins from any species. The positions of several additional motifs of potential biological significance also are indicated. (c) Dendrogram (Genetics Computer Group pileup program) indicating the relative similarities between Orc1- and Cdc6-related proteins from fungal and vertebrate species.