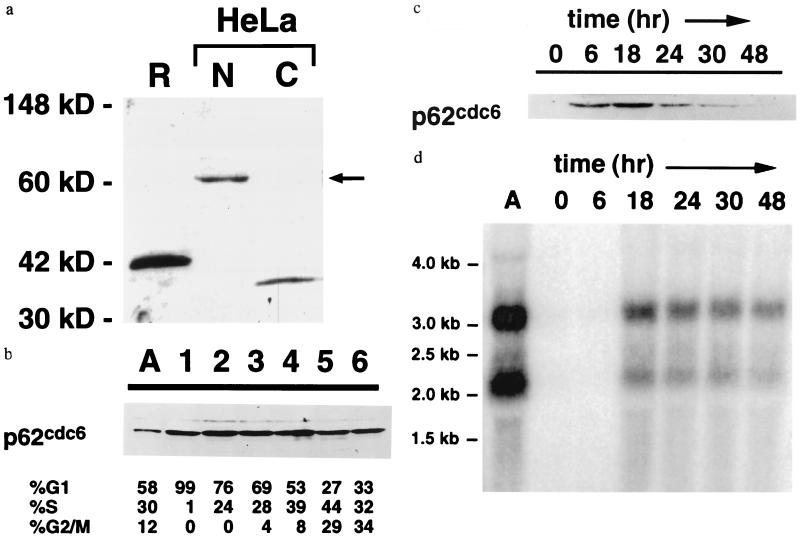
Figure 3.
(a) Nuclear localization of p62cdc6 in HeLa cells. Specific antisera raised against a recombinant, truncated form of p62cdc6 (R) expressed in bacteria identified a nuclear protein of 62 kDa in nuclear extracts (N, 20 μg) but not cytoplasmic extracts (C, 50 μg) of asynchronously growing human HeLa cells. (b) Expression of p62cdc6 in asynchronous HeLa cells (A) and in fractions of HeLa cells separated by centrifugal elutriation. Flow cytometry results are indicated. (c) Expression of p62cdc6 in quiescent and proliferating human diploid fibroblasts (Wi38 cells). Cells were grown at low density in the absence of serum for 48 hr and harvested at the indicated times following the addition of serum. The timing of entry into S phase varied among individual experiments, but flow cytometry indicated that the majority of cells (58–70%) consistently were in S phase between 18 and 24 hr after the addition of serum, and this percentage declined to less than 27% as growth became asynchronous. In b and c, each lane was loaded with 32 μg of nuclear protein, and equivalent protein loading was confirmed by staining total proteins with Commassie brilliant blue. Elutriation and serum stimulation experiments were repeated three or more times with similar results, except that expression of p62cdc6 in Wi38 cells at the 6-hr time point following serum stimulation was variable and not detectable in some experiments. (d) Northern blot indicating the abundance of p62cdc6 mRNA transcripts in asynchronous HeLa cells (A) and in Wi38 cells subjected to serum starvation and refeeding, as in c. Lanes were loaded with 15 μg total RNA and equivalent loading was confirmed by staining of ribosomal RNA subunits with ethidium bromide.