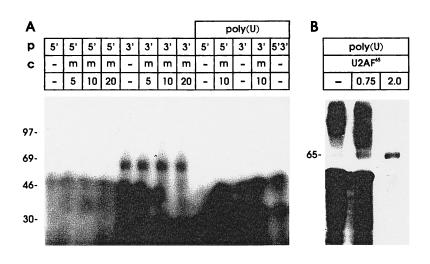
Figure 4.
Binding properties of the 65-kDa protein and its target site within the NRE. (A) HeLa cell nuclear proteins were UV-crosslinked to probes corresponding to the 5′ half of the NRE (probe 5′) with four 5′ splice site homologies, or the 3′ half of the NRE (probe 3′) with a G+U-rich region with homology to a U2AF65 binding site. Poly(U) denotes nuclear extracts depleted by preincubation with poly(U) Sepharose in the presence of 2 M KCl. Unlabeled RNA corresponding to the middle section of the NRE (m, see Fig. 2) was used as competitor. (B) Reconstitution experiment with U2AF65. The indicated amounts (in micrograms) of partially purified, bacterial protein extract containing U2AF65 were added to HeLa cell nuclear extracts that were depleted of 65-kDa protein binding activity by preincubation with poly(U) Sepharose in 2 M KCl, probe N was used (Fig. 2).