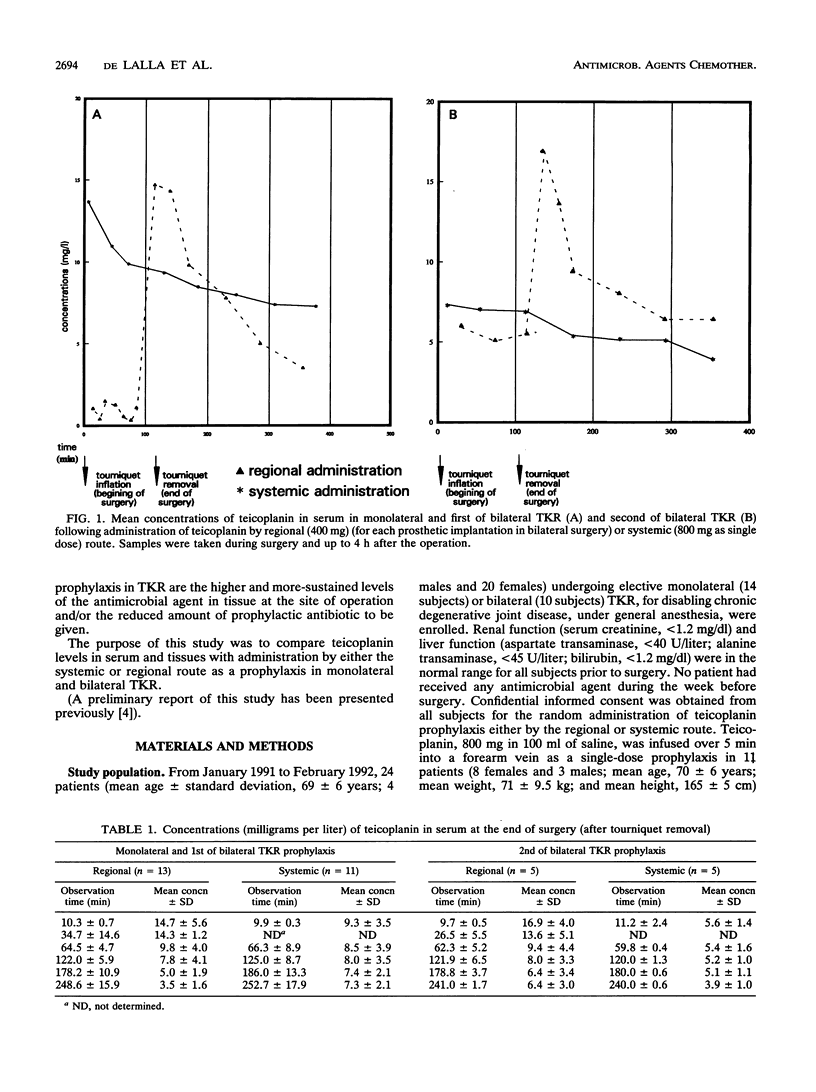
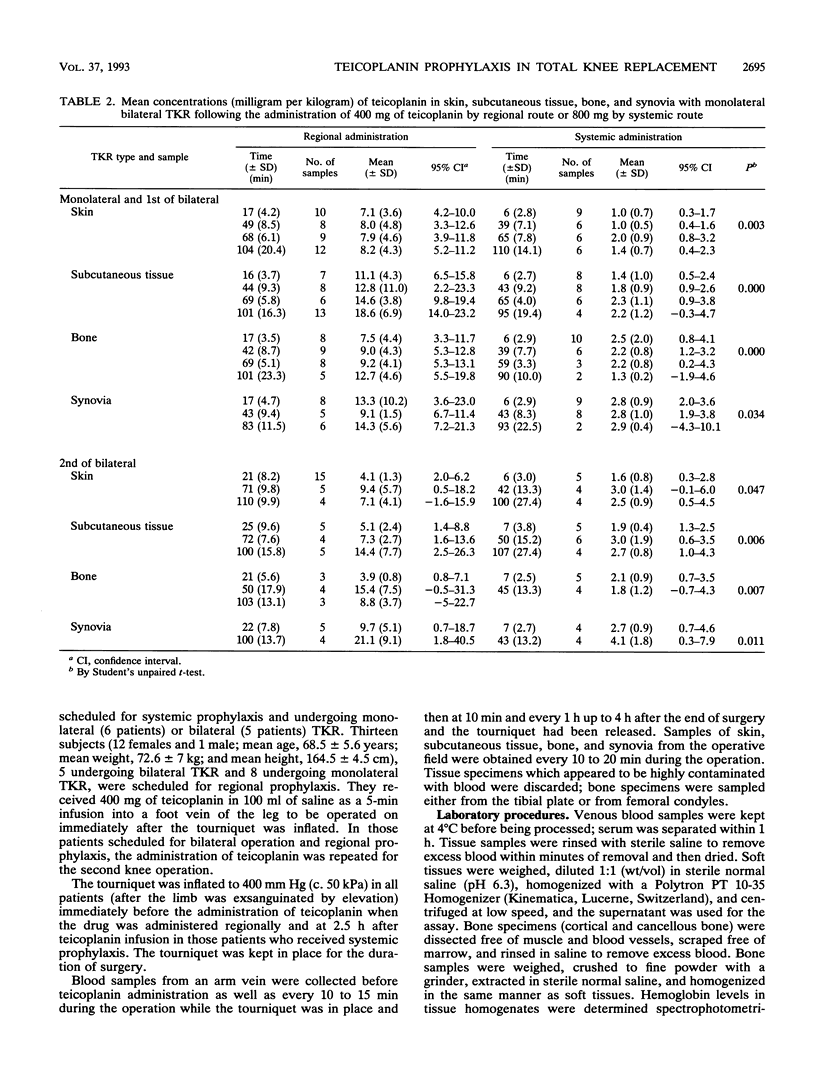
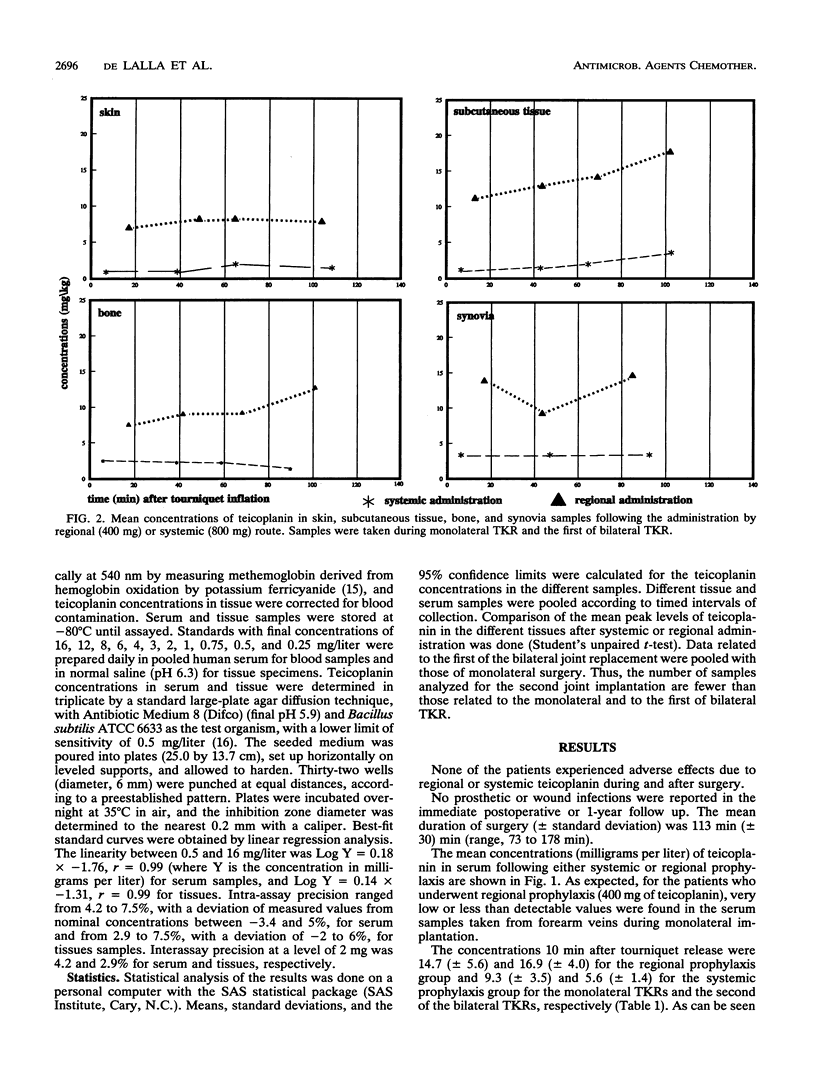
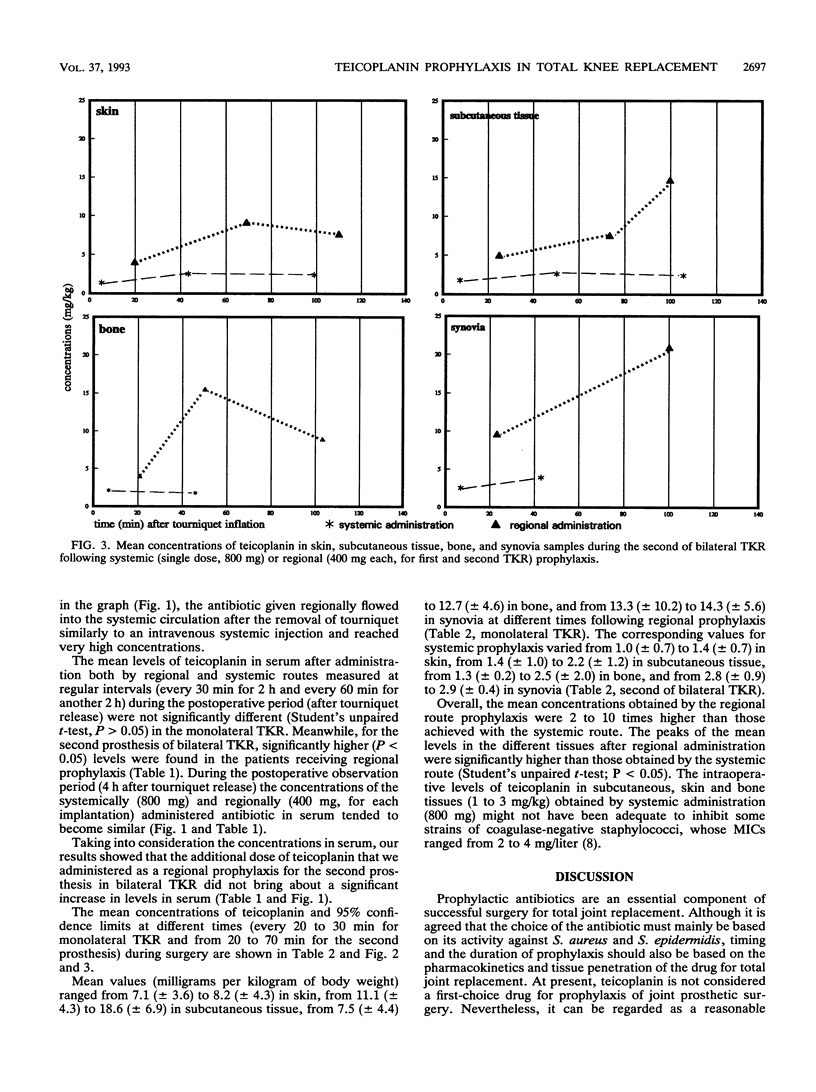
Abstract
Twenty-four patients undergoing monolateral or bilateral total knee replacement (TKR) procedures were randomized to receive teicoplanin (T) either systemically or regionally. Subjects scheduled for systemic prophylaxis and undergoing monolateral (six patients) or bilateral (five patients) TKR received a single 800-mg dose of T in 100 ml of saline as a 5-min infusion into a forearm vein 2.5 h before surgery. For regional prophylaxis, patients undergoing monolateral surgery (eight subjects) received 400 mg of T in 100 ml of saline as a 5-min infusion into a foot vein of the leg to be operated on immediately after the tourniquet was inflated. For the five patients scheduled for bilateral operation and regional prophylaxis, the administration of T was also repeated for the second knee operation. The tourniquet, as the standard TKR surgical technique, was inflated to 400 mm Hg (c. 50 kPa) in all 24 patients immediately before the beginning of surgery and kept in place for the duration of the operation. Samples of serum, bone, skin, synovia, and subcutaneous tissue were collected at timed intervals during surgery. They were microbiologically assayed for T by using Bacillus subtilis as the test organism. Overall, the mean T concentrations obtained with regional route prophylaxis were found to be 2 to 10 times higher than those achieved following systemic prophylaxis. Moreover, peak levels in different tissues after regional prophylaxis were significantly higher (P < 0.05). None of the patients experienced adverse effects due to regional or systemic T administration; no prosthetic or wound infections were observed in the follow-up period (from 12 to 26 months).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campoli-Richards D. M., Brogden R. N., Faulds D. Teicoplanin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic potential. Drugs. 1990 Sep;40(3):449–486. doi: 10.2165/00003495-199040030-00007. [DOI] [PubMed] [Google Scholar]
- Exner K., Lang E., Borsche A., Lemperle G. Efficacy, tolerability and pharmacokinetics of teicoplanin in patients undergoing breast surgery. Eur J Surg Suppl. 1992;(567):33–38. [PubMed] [Google Scholar]
- Gorbach S. L., Condon R. E., Conte J. E., Jr, Kaiser A. B., Ledger W. J., Nichols R. L. Evaluation of new anti-infective drugs for surgical prophylaxis. Infectious Diseases Society of America and the Food and Drug Administration. Clin Infect Dis. 1992 Nov;15 (Suppl 1):S313–S338. doi: 10.1093/clind/15.supplement_1.s313. [DOI] [PubMed] [Google Scholar]
- Greenwood D. Microbiological properties of teicoplanin. J Antimicrob Chemother. 1988 Jan;21 (Suppl A):1–13. doi: 10.1093/jac/21.suppl_a.1. [DOI] [PubMed] [Google Scholar]
- Hoddinott C., Lovering A. M., Fernando H. C., Dixon J. H., Reeves D. S. Determination of bone and fat concentrations following systemic cefamandole and regional cefuroxime administration in patients undergoing knee arthroplasty. J Antimicrob Chemother. 1990 Dec;26(6):823–829. doi: 10.1093/jac/26.6.823. [DOI] [PubMed] [Google Scholar]
- LeFrock J. L., Ristuccia A. M., Ristuccia P. A., Quenzer R. W., Haggerty P. G., Allen J. E., Lettau L. A., Schwartz R., Appleby D. Teicoplanin in the treatment of bone and joint infections. Teicoplanin Bone and Joint Cooperative Study Group, USA. Eur J Surg Suppl. 1992;(567):9–13. [PubMed] [Google Scholar]
- Marone P., Concia E., Andreoni M., Suter F., Cruciani M. Treatment of bone and soft tissue infections with teicoplanin. J Antimicrob Chemother. 1990 Mar;25(3):435–439. doi: 10.1093/jac/25.3.435. [DOI] [PubMed] [Google Scholar]
- Norden C. W. Antibiotic prophylaxis in orthopedic surgery. Rev Infect Dis. 1991 Sep-Oct;13 (Suppl 10):S842–S846. doi: 10.1093/clinids/13.supplement_10.s842. [DOI] [PubMed] [Google Scholar]
- Paya C. V., Wilson W. R., Fitzgerald R. H., Jr Management of infection in total knee replacement. Curr Clin Top Infect Dis. 1988;9:222–240. [PubMed] [Google Scholar]
- Seidel C., Richter U. G., Bühler S., Hornstein O. P. Drug therapy of diabetic neuropathic foot ulcers: transvenous retrograde perfusion versus systemic regimen. Vasa. 1991;20(4):388–393. [PubMed] [Google Scholar]
- Verbist L., Tjandramaga B., Hendrickx B., Van Hecken A., Van Melle P., Verbesselt R., Verhaegen J., De Schepper P. J. In vitro activity and human pharmacokinetics of teicoplanin. Antimicrob Agents Chemother. 1984 Dec;26(6):881–886. doi: 10.1128/aac.26.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A. P., Taylor B., Treasure T., Grüneberg R. N., Patton K., Felmingham D., Sturridge M. F. Antibiotic prophylaxis in cardiac surgery: serum and tissue levels of teicoplanin, flucloxacillin and tobramycin. J Antimicrob Chemother. 1988 Feb;21(2):201–212. doi: 10.1093/jac/21.2.201. [DOI] [PubMed] [Google Scholar]
- van KAMPEN E., ZIJLSTRA W. G. Standardization of hemoglobinometry. II. The hemiglobincyanide method. Clin Chim Acta. 1961 Jul;6:538–544. doi: 10.1016/0009-8981(61)90145-0. [DOI] [PubMed] [Google Scholar]


