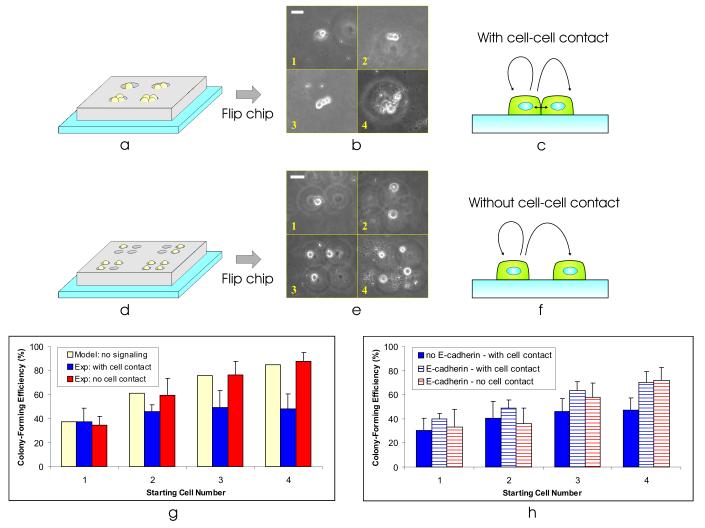
Figure 5.
Precise control of the stem cell microenvironment. (a) By making the well dimensions large enough to trap multiple cells, we can vary the number of cells patterned at each spot. (b) Using a BFC with microwell-to-microwell spacing of 0.75 mm, well diameter of 40 μm, and well height of 50 μm, we patterned clusters of cells with 1-4 cells at each spot. Collage is from images taken randomly from different areas on the substrate. Scale bar, 40 μm. (c) Patterning the cells as clusters allows them to experience cell-cell contact. (d) Using multiple single-cell sized wells, we can pattern small groups of cells without any cell-cell contact. (e) Using a BFC with microwell-to-microwell spacing of 0.75 mm, well diameter of 30 μm, and well height of 30 μm, we patterned small groups of 1-4 single mESCs at each spot. Using microwells with edge-edge distance of ∼20 μm (n = 30), we patterned the cells with a membrane-membrane distance of 45 ± 15 μm (n = 33). Collage is from images taken randomly from different areas on the substrate. Scale bar, 40 μm. (f) Patterning mESCs as small groups of single cells allows us to turn off cell-cell contact. (g) Colony-forming efficiency (2 days after cell seeding) as a function of starting cell number for three cases – model of no cell-cell signaling (solid yellow bars), experiment with cell-cell contact (solid blue bars), experiment without cell-cell contact (solid red bars). Bars on experimental data indicate 1 standard deviation from the mean. (h) Colony-forming efficiency as a function of starting cell number for three cases – cells in contact without exposure to anti-E-cadherin (solid blue bars), cells in contact with exposure to anti-E-cadherin (striped blue bars), and cells without contact with exposure to anti-E-cadherin (striped red bars). Bars on experimental data indicate 1 standard deviation from the mean.