Figure 4.
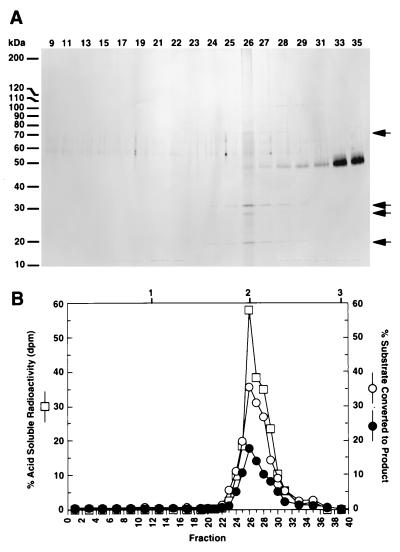
Junction RNase activity is tightly associated with RNase HI. (A) An aliquot of purified RNase HI was subjected to sedimentation on a 15–35% glycerol gradient. After fractionation, aliquots were analyzed by electrophoresis on an 8–14% SDS-polyacrylamide gel and stained with silver. Gel bands corresponding to RNase HI are indicated (arrows). The peak of RNase HI is fraction 26 that corresponds to a sedimentation value of ≈4.3 and a native molecular mass of ≈68 kDa as expected for RNase HI. (B) Glycerol gradient fractions (diluted 1:400) were tested for activity on [3H]RNA/DNA hybrids and Okazaki substrates as previously described. The percent of acid soluble radioactivity (□) and the percent of double-stranded (•) and single-stranded (○) Okazaki substrate to converted to product were plotted vs. fraction number. Above the graph, positions of protein standards for the glycerol gradient are: (1) RNase (13.7 kDa), (2) BSA (68 kDa), and (3) catalase (232 kDa).