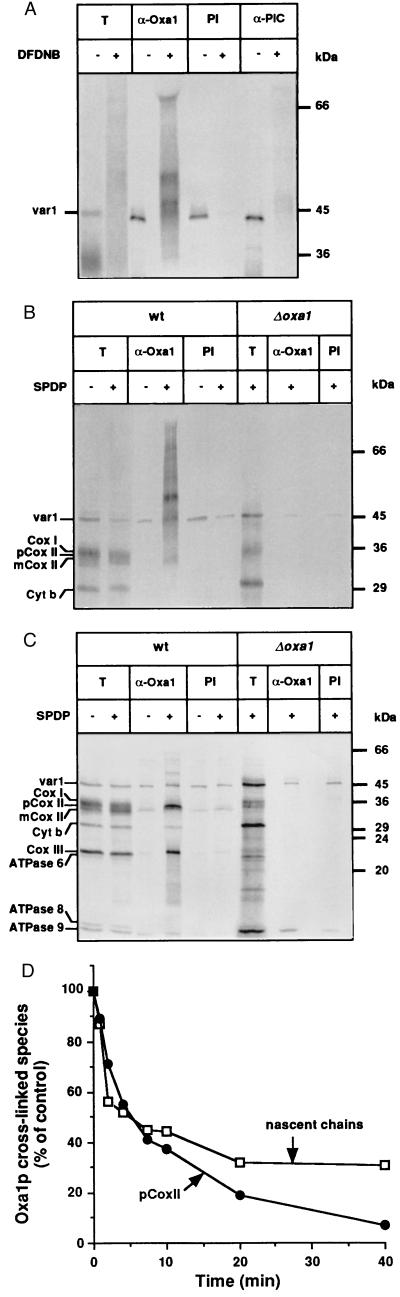
Figure 3.
Oxa1p interacts cotranslationally with mitochondrially encoded proteins. (A) Oxa1p is in direct contact with nascent chains of mitochondrial translation products. In organello translation was performed for 15 min at 25°C in wild-type mitochondria in the presence of [35S]methionine (3). Samples were divided and incubated with the chemical cross-linker DFDNB (0.2 mM) or were mock-treated with buffer lacking the cross-linker for 15 min at 25°C. Cross-linking and labeling were stopped by the addition of glycine, unlabeled methionine, and puromycin. Mitochondria were reisolated, washed, and lysed. Solubilized cross-linked products were immunoprecipitated with either preimmune serum (PI) or antiserum specific for Oxa1p (α-Oxa1) (4) or the phosphate carrier (α-PiC). T, 10% of the total solubilized material used for immunoprecipitation. (B and C) Oxa1p interacts not only with nascent chains but also with fully synthesized translation products. In organello translation and cross-linking of mitochondrial translation products with the cleavable cross-linker SPDP (0.3 mM) were performed in mitochondria isolated from Δoxa1 and its corresponding wild type (wt), as described above. Immunoprecipitation of cross-linked products was performed with preimmune serum (PI) or Oxa1p-specific antiserum (α-Oxa1). Samples were divided and analyzed by SDS/PAGE with sample buffer without 2-mercaptoethanol (B) or containing 2-mercaptoethanol (C). T, 2% and 10% of total solubilized material from the wild-type and Δoxa1 mitochondria, respectively, which were used for immunoprecipitation reactions. (D) Nascent chains and pCoxII interact with Oxa1p in a transient manner. In organello translation was performed for 15 min at 25°C in wild-type mitochondria in the presence of [35S]methionine, after which an excess of unlabeled methionine (10 mM) was added, and samples were incubated further at 25°C for the times indicated. Cross-linking with SPDP was carried out as described above. Immunoprecipitation of cross-linked products was performed with Oxa1p-specific antiserum, and samples were analyzed by SDS/PAGE in the absence of 2-mercaptoethanol (for quantitation of nascent chains) or in the presence of 2-mercaptoethanol (for quantitation of pCoxII). The amounts of nascent chains and pCoxII that could be cross-linked with Oxa1p were quantified by phosphorimaging; they are expressed for both species as a percent of their amount associated with Oxa1p at the beginning of the chase period.