Figure 1.
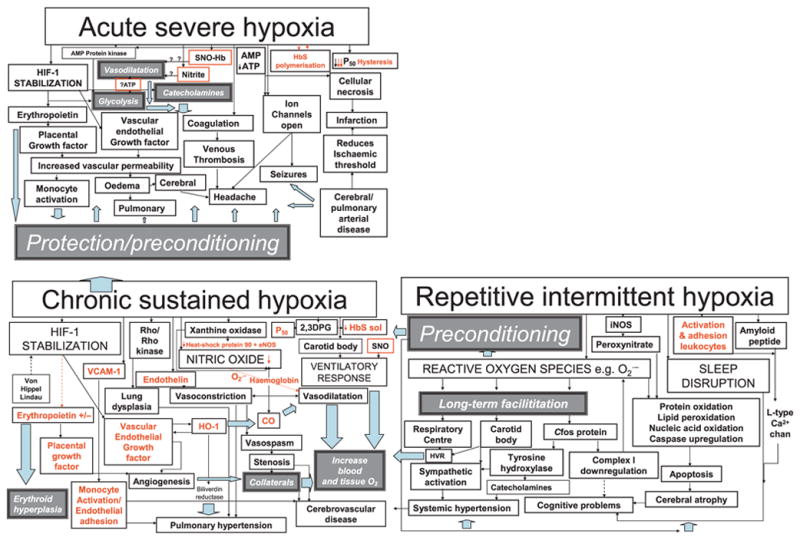
Clinical features are represented by pale grey boxes while white boxes/black arrows refer to mechanisms likely to lead to pathology (red for those for which there is evidence in sickle cell disease, SCD) and dark grey boxes/pale blue arrows refer to potentially protective mechanisms. Red boxes denote mechanisms involving the red cell postulated to be involved in vasodilatation in response to local hypoxia. In chronic sustained hypoxia, hypoxia-inducible factor stabilization leads to upregulation of erythropoietin and growth factors. In addition, there is increased nitric oxide synthesis, although in SCD free haemoglobin secondary to chronic haemolysis and the superoxide generated by increased xanthine oxidase activity may reduce bioavailability and tip the delicate balance between vasodilatation and vasoconstriction in favour of the latter. Vasconstriction is also favoured by the release of endothelin in response to hypoxia. Increased 2,3 dihydophosphoglycerate (2,3 DPG) further increases P50 and decreases Haemoglobin S solubility. Adhesion molecules, such as VCAM-1, are upregulated and favour monocyte adhesion to the endothelium. Upregulation of haem-oxygenase-1 (HO-1) may have protective effects, including vasodilatation and reduction of P50 secondary to the generation of carboxyhaemoglobin, and the antioxidant effects of bilirubin reductase, but also further upregulates vascular endothelial growth factor and may increase carbon monoxide levels locally. The ventilatory response may be increased, probably facilitated by repetitive intermittent hypoxia. Cerebral infarction, atrophy and cognitive problems may be related to a number of mechanisms related to chronic sustained and intermittent hypoxia, including perhaps demyelination secondary to chronically high carbon monoxide levels, the adverse effects of reactive oxygen species generated by repetitive intermittent hypoxia on proteins, nucleic acids and lipids, downregulation of the mitochondrial respiratory chain enzymes and upregulation of amyloid β peptide, as well as the effect of sleep disruption. Chronically hypoxic patients with SCD may be preconditioned and therefore relatively protected from the effects of acute hypoxia, e.g. vasogenic oedema secondary to increased vascular endothelial growth factor, opening of ion channels and venous thrombosis. However, the very rapid polymerization of HbS may cause such severe hypoxia that immediately available compensatory mechanisms, such as increased glycolysis, relative hypertension secondary to catecholamine release, and vasodilatation secondary to release of nitric oxide by S-nitrosylated haemoglobin (SNO-Hb), are overwhelmed and acute neurological complications, such as headache, seizures, cerebral oedema, as well as stroke, are inevitable.
