Abstract
Background and Purpose
Shockwave lithotripsy (SWL) has become a first-line intervention for treatment of nephrolithiasis. However, few studies have examined the effects of modifications in the method of shockwave energy administration on comminution efficiency. We propose that a gradual increase in output voltage will produce superior stone fragmentation in comparison with a constant or a decreasing output voltage by optimizing the stress wave and cavitation erosion forces on renal calculi.
Materials and Methods
BegoStone® phantoms were implanted in the renal pelvis of 11 pigs that underwent SWL at a pulse repetition rate of 1 Hz. Animals in the increasing strategy group (N = 4) were subjected to 18, 20, and 22 kV for 600, 600, and 800 shocks, respectively. The second group (N = 4) received a decreasing strategy of 22, 20, and 18 kV for 800, 600, and 600 shocks, respectively. The third group (N = 3) received all 2000 shocks at 20 kV, mimicking the clinical protocol.
Results
A progressively decreasing strategy and constant output voltage produced a mean comminution efficiency, or percentage of stone fragments <2 mm, of 89.0% ± 3.3% and 87.6% ± 1.7%, respectively. The mean comminution efficiency was improved to 96.5% ± 1.4% by using the increasing strategy (P = 0.01).
Conclusions
A progressive increase in lithotripter output voltage during SWL can produce greater stone fragmentation than protocols employing constant or decreasing output voltage.
INTRODUCTION
Despite the widespread use of shockwave lithotripsy (SWL) for the treatment of nephrolithiasis, few studies have examined the effects of modifications to the method of energy administration. The strategy currently employed in the clinical setting uniformly applies a constant output voltage to a target stone for 2000 shocks, although this technique may vary according to the type of lithotripter, stone size, and stone composition. It would be of great clinical benefit to elucidate whether varying treatment strategies result in different rates of stone comminution. In-vitro studies at our institution suggested that treatment strategies impact the degree of stone fragmentation, with a progressive increase in output voltage yielding the most efficient comminution.1
In this study, an in-vivo porcine model was used to verify the effect of shockwave treatment strategy on stone comminution efficiency. We hypothesize that a gradual increase in output voltage will produce greater overall stone fragmentation than a gradual decrease in shockwave energy. These experimental groups are compared with animals treated with a constant output voltage, mimicking the current prevalent clinical setting.
MATERIALS AND METHODS
Animal preparation
After approval had been obtained from the Institutional Animal Care and Use Committee, 11 juvenile female pigs weighing 25.4 to 39.5 kg (mean 36.3 kg) were acquired for the study. The animals underwent induction of general endotracheal anesthesia with a combination of ketamine (2.2 mg/kg), acepromazine (0.2 mg/kg), and atropine (0.4 mg/kg). Anesthesia was maintained with inhalational isoflurane (1%–3%), which was titrated as needed. During the procedure, oxygen saturation, pulse, and ventilatory rate were monitored closely.
Surgical dissection through a standard midline laparotomy incision exposed the right kidney and the proximal portion of the right ureter. A No. 10 blade was used to make a ∼2-cm incision through the anterior wall of the proximal ureter. A previously weighed BegoStone® phantom (Bego USA, Smithfield, RI) measuring 6.8 mm in diameter and 11.4 mm in length was introduced into the lumen of the ureter and advanced into the renal pelvis in a retrograde fashion using lithotomy forceps. A 6F polyurethane internal ureteral stent was placed in the right ureter and renal pelvis. The ureterotomy was closed in one layer with a running suture. Radiopaque staples were placed in the renal fascia lateral, medial, and inferior to the lower pole of the kidney to facilitate fluoroscopic localization during SWL treatment. The midline incision was closed with a running 0 Maxon suture.
Shockwave lithotripsy
One hour after stone placement, the pigs were transported to the SWL laboratory, and the position of the stone phantom was confirmed with C-arm fluoroscopy (Stenoscope; GE Medical Systems). The animal was placed in a supine position in the gantry of a Dornier HM-3 lithotripter (Dornier MedTech, Kennesaw, GA) and immersed in the waterbath filled with degassed and deionized water (temperature 37°C). The implanted kidney stone was positioned at the focal point of the lithotripter with the aid of the biplanar fluoroscopic imaging system of the HM-3 machine (Fig. 1). During SWL treatment, the animals were repositioned every 150 shocks to align the largest residual fragment or fragment group in the lithotripter focus.
FIG. 1.
Alignment with lithotripter focus. Opaque stone can be seen under cross in one of biplanar fluoroscopic images. Stent and localization staples are visible below and to right of stone.
The pigs were randomly divided into three treatment groups of increasing, decreasing, or constant lithotripter-output voltage. The animals assigned to the increasing strategy of shock-wave administration (N = 4) were subjected to 18, 20, and 22 kV for 600, 600, and 800 shocks, respectively, for a total of 2000 shocks. The second treatment group (N = 4) received a decreasing strategy of 22, 20, and 18 kV for 800, 600, and 600 shocks, respectively. The third group (N = 3) received 2000 shocks at 20kV. The shockwave-repetition rate remained constant at 1 Hz (60/min) for all treatment groups.
Analysis of stone fragmentation
After the lithotripsy treatment, the animals remained under general anesthesia and were returned to the operating room. Bilateral nephrectomies were performed through the previous midline incision, and the animals were euthanized humanely. The kidneys were bisected to expose the renal pelvis and the residual stone fragments in the collecting system (Fig. 2). The fragmentation pattern was photographed. The residual fragments were then collected and stored.
FIG. 2.
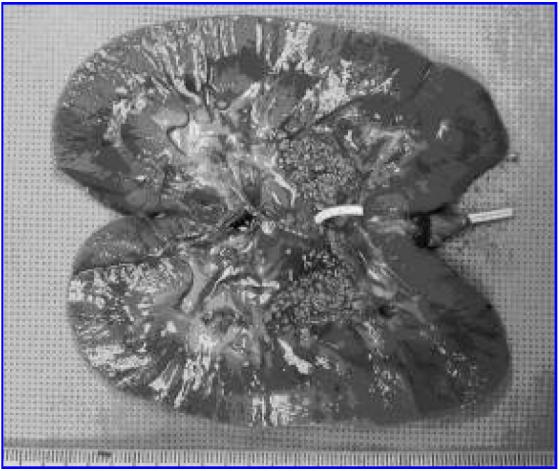
Bisected surface of kidney demonstrates fragmentation patterns produced during SWL.
For analysis, the residual fragments were allowed to air dry at room temperature for 24 hours and then filtered through a stack of standard sieves with decreasing mesh size of 4, 2, 1, and 0.5 mm. Stone comminution efficiency was determined by the percentage of fragments <2 mm. The mean efficiencies of stone comminution among the three groups were compared using ANOVA and Student's t-test (two-tailed).
RESULTS
Figure 3 demonstrates the stone-comminution efficiencies for all three protocols. The increasing treatment strategy, which started at 18 kV and progressed to 22 kV, produced a mean comminution efficiency of 96.5% ± 1.4%. Stones exposed to a decreasing treatment strategy, starting at 22 kV and proceeding to 18 kV, had a mean comminution of 89.0% ± 3.3%. At a constant output voltage of 20 kV, comminution efficiency was 87.6% ± 1.7%.
FIG. 3.
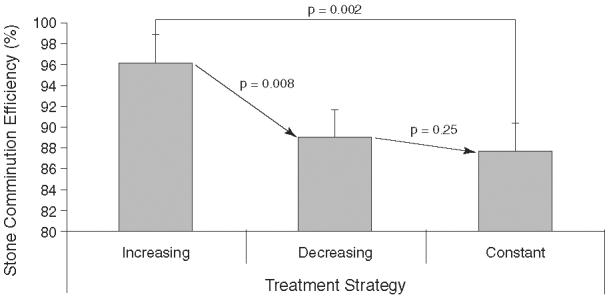
Stone comminution efficiency after SWL using the three treatment strategies: increasing from 18 to 20 and then to 22 kV, decreasing from 22 to 20 and then to 18 kV, and constant output voltage at 20 kV. All three were delivered at 1 Hz for 2000 shocks. Comminution efficiency was defined as percentage of fragments <2 mm; i.e., small enough to be voided in the urine.
Variation in treatment strategy thus produced a significant difference in mean comminution. Although each treatment strategy utilized the same or a similar dose of shockwave energy, the mean comminution efficiency produced by increasing output voltage was superior to that of decreasing (P = 0.016) and constant (P = 0.005) output voltages. The degree of stone fragmentation was not different between the decreasing and constant output-voltage groups (P = 0.54).
DISCUSSION
In this in-vivo study, we demonstrate that maximum stone-comminution efficiency depends on the method of shockwave energy administration in addition to the total energy delivered. To our knowledge, there are no published in-vivo studies that have examined modifications to the output voltage of the shock-wave protocol. This work compared the overall efficiency of shockwave protocols that used a stepwise increase or decrease or constant output voltage.
In the current investigation, a progressive increase in lithotripter voltage generated the greatest stone-comminution efficiency, which is consistent with previous in-vitro studies performed in our laboratory comparing these same strategies.1 Similar findings were reported in another study using lower output voltages and gypsum stones in vitro.2 However, gypsum stones are not as dense and hard as the BegoStone® phantoms, which are analogous to the calcium oxalate monohydrate stones most commonly observed in stone patients.3,4 Moreover, our goal was to use an acoustic dose of shockwave energy that was comparable to that employed in the clinical setting. Therefore, the constant-output strategy was not performed at voltages <20 kV. The total acoustic dosage of each protocol utilized in the current study is similar to that used on the conventional Dornier HM-3 lithotripter.
The treatment strategies described herein differ in their utilization of stress wave and cavitation forces. Stress waves and cavitation have been shown to work synergistically, instead of independently, to produce stone fragmentation.5 The stress waves can initiate fragmentation at low output voltages, but higher-energy shockwaves are required to produce greater cavitation activity and overcome the attenuation and scattering effect created by the collection of stone fragments.1,5 In the later part of SWL therapy, increased output voltages compensate for attenuation created by the collection of small particles surrounding the larger residual stone fragments and also enhance the cavitation activity. In contrast, decreasing output voltages will produce a high initial fragmentation rate; however, the accumulation of small particles will increase the attenuation of subsequent shockwaves and thus decrease the effectiveness of stress waves and cavitation erosion in the later portion of the treatment.1
In our efforts to improve minimally invasive stone fragmentation, it is imperative that we also consider the potential for adverse tissue effects. Animal and clinical studies have established that SWL therapy is often accompanied by renal injury, including hematuria, subcapsular hematomas, and diffuse parenchymal hemorrhage.6-8 Stress waves and cavitation forces are thought to contribute to SWL-induced renal injury, as manifested by gross morphologic damage, as well as impairment of renal blood flow and glomerular filtration rate.6-9 Cavitation bubbles have been shown in vitro and in vivo to be one cause of blood-vessel damage.10,11 If the tensile energy of the shock-wave is large enough, intraluminal cavitation bubbles will expand over that time interval, dilate the vessel wall, and subsequently lead to the rupture of the blood vessel.11,12 It therefore would be preferable to avoid the creation and collapse of cavitation bubbles at the outset.
Initial shockwaves administered at low output voltages can curtail the intraluminal bubble expansion and thus avoid vessel rupture. Furthermore, the initial low pressures may provoke vaso-constriction, leading to a reduction in the intraluminal volume of blood and the number of cavitation bubble nuclei.13 These two factors will diminish the propensity for renal-parenchymal injury at subsequent higher pressures. One such study demonstrated that treatment of the kidney with 100 to 500 low-energy shockwaves reduces subsequent injury to the renal parenchyma from an additional 2000 shocks at a high output voltage.13 Given these data, further in-vivo studies to evaluate the effects of various treatment strategies on renal injury are warranted.
It would be simple to implement an optimized treatment strategy as a means of maximizing stone comminution efficiency. This modification can be adapted quickly to the current clinical lithotripters within the output range approved by the Food and Drug Administration. It should be noted that although this study demonstrates the potential for improvement, the optimal treatment strategy that can lead to maximum stone comminution with minimal tissue injury needs to be determined in future studies.
CONCLUSIONS
We have demonstrated that efficient stone comminution depends on the method of shockwave energy administration. A treatment strategy that utilizes a progressive increase in lithotripter voltage produced greater comminution efficiency than protocols that employed a constant or decreasing voltage output. Our results suggest that a progressive increase in lithotripter output voltage may be considered for use in the clinical setting as a safe and effective treatment strategy.
ACKNOWLEDGMENTS
This project was supported by National Institutes of Health Grants R01-DK52985 and R01-DK58266 and a Howard Hughes Medical Institutes Medical Student Research Fellowship.
ABBREVIATIONS USED
- ANOVA
analysis of variance
- Hz
Hertz
- kV
kilo-volts
- SWL
shockwave lithotripsy
REFERENCES
- 1.Zhou YF, Cocks FH, Preminger GM, Zhong P. Treatment strategy affects stone comminution efficiency in shock wave lithotripsy. J Urol. 2004;172:349. doi: 10.1097/01.ju.0000132356.97888.8b. [DOI] [PubMed] [Google Scholar]
- 2.McAteer JA, Baird T, Williams JC, Jr, Hatt EK, Evan AP, Cleveland RO. Voltage-stepping during SWL influences stone breakage independent of total energy delivered: In vitro studies with model stones [abstract 1825]; Presented at the American Urological Association Annual Meeting; Chicago, Il. 2003. [Google Scholar]
- 3.Liu Y, Zhong P. BegoStone: A new phantom for shock wave lithotripsy research. J Acoust Soc Am. 2002;112:1265. doi: 10.1121/1.1501905. [DOI] [PubMed] [Google Scholar]
- 4.Heimbach D, Munver R, Zhong P, et al. Acoustic and mechanical properties of artificial stones in comparison to natural kidney stones. J Urol. 2000;164:537. [PubMed] [Google Scholar]
- 5.Zhu S, Cocks FH, Preminger GM, Zhong P. The role of stress waves and cavitation in stone comminution in shock wave lithotripsy. Ultrasound Med Biol. 2002;28:661. doi: 10.1016/s0301-5629(02)00506-9. [DOI] [PubMed] [Google Scholar]
- 6.Lokhandwalla M, Sturtevant B. Fracture mechanics model of stone comminution in ESWL and implication for tissue damage. Phy Med Biol. 2000;45:1923. doi: 10.1088/0031-9155/45/7/316. [DOI] [PubMed] [Google Scholar]
- 7.Shao Y, Connors BA, Evan AP, Willis LR, Lifshitz DA, Lingeman JE. Morphological changes induced in the pig kidney by extracorporeal shock wave lithotripsy: Nephron-injury. Anat Rec. 2003;275A:979. doi: 10.1002/ar.a.10115. [DOI] [PubMed] [Google Scholar]
- 8.Willis LR, Evan AP, Connors BA, Fineberg NS, Lingeman JE. Effects of SWL on glomerular filtration rate and renal plasma flow in uninephrectomized minipigs. J Endourol. 1997;11:27. doi: 10.1089/end.1997.11.27. [DOI] [PubMed] [Google Scholar]
- 9.Gambihler S, Delius M, Brendel W. Biological effects of shock waves: Cell disruption, viability, and proliferation of L1210 cells exposed to shock waves in vitro. Ultrasound Med Biol. 1990;16:587. doi: 10.1016/0301-5629(90)90024-7. [DOI] [PubMed] [Google Scholar]
- 10.Knapp PM, Kulb TB, Lingeman JE, et al. Extracorporeal shock wave lithotripsy-induced perirenal hematomas. J Urol. 1988;139:700. doi: 10.1016/s0022-5347(17)42604-8. [DOI] [PubMed] [Google Scholar]
- 11.Zhong P, Cioanta I, Zhu S, Cocks FH, Preminger GM. Effects of tissue constraint on shock wave-induced bubble expansion in vivo. J Acoust Soc Am. 1998;104:3126. doi: 10.1121/1.423905. [DOI] [PubMed] [Google Scholar]
- 12.Zhong P, Zhou Y, Zhu S. Dynamics of bubble oscillation in constrained media and mechanisms of vessel rupture in SWL. Ultrasound Med Biol. 2001;27:119. doi: 10.1016/s0301-5629(00)00322-7. [DOI] [PubMed] [Google Scholar]
- 13.Willis LR, Evan AP, Connors BA, Blomgren PM, Handa RK, Lingeman JE. Same-pole application of low- and high-energy shock waves protects kidney from SWL-induced tissue injury [abstract 1114]; Presented at the American Urological Association Annual Meeting; San Francisco. 2004. [Google Scholar]


