Abstract
Objectives
A G>T transversion in a tyrosine kinase JAK2 (V617F) was reported in over 80% of patients with polycythemia vera (PV). Current evidence suggests that JAK2V617F somatic mutation is involved in the pathogenesis of PV, since it confers erythropoietin independent proliferation to erythroid progenitor cells. However, several unanswered questions regarding the essential role of JAK2V617F arose as a) it is not a dominant mutation, b) it is not PV specific since it is found in several myeloproliferative disorders and c) some (~20%) PV patients lack the JAK2V617F mutation. We set up to investigate the relative frequency of JAK2V617F in in vitro expanded PV progenitors.
Methods
In vitro expansion of erythroid progenitors from mononuclear cells was optimized. Frequency of JAK2V617F allele was measured by using allele-specific real-time PCR. Clonality was performed using established procedure.
Results
In vitro expansion of PV erythroid progenitors and differentiated dendritic cells resulted in a decrease of the frequency of JAK2V617F allele compared to granulocytes or CD235+ erythroid progenitors. Clonality analysis demonstrated that while granulocytes of these PV patients were clonal, expanded erythroid cells were polyclonal. However, in vitro expanded PV erythroid progenitors still had ~two fold increased proliferative capacity in comparison to erythroid progenitors from healthy individuals. Erythropoietin favors the cells without JAK2V617F allele. Dendritic cells in one out of three patients remained clonal.
Conclusion
JAK2V617F mutation does not provide a proliferative/survival advantage to the PV clone during in vitro expansion. These data suggest that the JAK2V617F mutation plays an important role in the biology of PV, yet it may not be the PV-initiating event.
Introduction
Polycythemia vera (PV), essential thrombocythemia, idiopathic myelofibrosis, and chronic myelogenous leukemia (CML) are chronic myeloproliferative disorders (MPD) distinguished by clonal hematopoiesis1. Unlike CML, which is characterized by the t (9;22) translocation, in other myeloproliferative disorders, a specific cytogenetic marker is not present2. Our group using DNA microsatellite markers identified a loss-of-heterozygosity of chromosome 9p in ~30% of PV patients resulting from uniparental disomy3. This has provided the basis for the discovery of a single nucleotide mutation (G1849T) in JAK2 located in chromosome 9p that is present in the overwhelming majority of PV patients; either as a single allele, or converted to homozygosity by uniparental disomy4–8. JAK2V617F is a gain-of-function mutation that leads to constitutive tyrosine phosphorylation of JAK2 and activation of its downstream transcription factors4.
Imatinib, an inhibitor of the Bcr-Abl tyrosine kinase activity9, showed an impressive therapeutic efficacy in CML. Imatinib inhibits several other tyrosine kinases, such as c-KIT, TEL-PDFGR, FIP1L1-PDGFR and COL-PDGF10. We have previously demonstrated selective inhibition of mouse FDCP reporter cells transfected with JAK2 1849G>T, albeit only with high imatinib concentration11. Moreover, native in vitro expanded erythroid progenitors from PV patients were more sensitive to imatinib than the mouse reporter cells11, (and Gaikwad et al. revised manuscript under review, Exp. Hem.) suggesting a biological difference between transfected reporter cells and native PV cells.
To further elucidate the molecular mechanism of imatinib, we sought to evaluate and correlate the frequency of JAK2V617F in in vitro expanded native PV erythroid progenitors with their response to imatinib. Here we demonstrate both, a decrease in the frequency of cells expressing JAK2V617F and a conversion to polyclonal erythropoiesis during in vitro expansion of PV progenitors12. We propose that the JAK2V617F mutation does not provide a proliferative/survival advantage to the PV clone during in vitro expansion and that some other factors may account for increased expansion of PV progenitors.
Materials and Methods
Reagents
Lymphocyte separation medium was obtained from Mediatech (Herndon, VA); Insulin growth factor 1 (IGF-1), Prostaglandin E2, protease inhibitors sodium orthovanadate and sodium fluoride were purchased from Sigma Chemical Co (St. Louis, MO); cytokine cocktail (CC110: 100X stock containing 10 μg/ml of fetal liver tyrosine kinase 3 ligand, rh-thrombopoietin and rh-stem cell factor including) and Stem Factor Cell medium used for expansion, were bought from Stem Cell Technologies (Vancouver, Canada). Erythropoietin (Epo) was purchased from Amgen (Thousand Oaks, CA); recombinant human stem cell factor (hSCF) and human IL-4 were obtained from R&D systems (Minneapolis, MN). CellGenix media was purchased from CellGenix USA (Antioch, IL) and human GM-CSF from Immunex Corp. (Seattle, WA). Protein estimation was done using Bradford reagent from BioRad, (Hercules, CA); the red cell lysis buffer was obtained from Promega (Madison, WI); DNAzol extraction kit and Trizol reagent was purchased from Invitrogen (Carlsbad, CA). TaqMan Universal PCR master mix, JAK2 universal forward and allele-specific reverse primers; FAM labeled JAK2, MGB probe was purchased from Applied Biosystems (Foster City, CA).
Antibodies for flow cytometry and immunoblot analysis
Phycoerythrin (PE)-conjugated anti-CD235A (glycophorin) and fluorescein isothiocyanate (FITC)-conjugated anti-human-CD71 (transferrin receptor) monoclonal antibodies were from BD Biosciences (San Jose, CA). Anti-Bclxl antibodies were purchased from Santa Cruz (Santa Cruz, CA). Anti-caspase3 and β-actin antibodies were from Sigma (St. Louis MO).
In vitro expansion of human erythroid progenitors
Blood specimens from the PV patients and healthy donors (controls) were obtained with consent on an Institutional Review Board (IRB) approved protocol. The mononuclear cell population was isolated from whole blood using standard protocols3. Expansion of the progenitor cells from the mononuclear cell population was performed in three steps based on our modification of published protocol13. In the first step (days 0–7), 3 × 105/ml mononuclear cells were cultured in the StemSpan™ Serum-Free Expansion Medium in the presence of the cytokine cocktail containing 100ng/ml of fetal liver tyrosine kinase 3 ligand, 100ng/ml of thrombopoietin, and 100ng/ml of stem cell factor. In the second step (days 8–14), the cells obtained on day 7 were re-suspended at 5 ×105/ml in the same medium containing 50ng/ml of stem cell factor, 50ng/ml of insulin like growth factor-1, and 3U/ml of erythropoietin. In the third step, the cells collected on day 14 were re-suspended at 106/ml and cultured for a further two to seven days in the presence of the same cytokine mixture as in the previous step, but without stem cell factor. The cultures were incubated at 37°C in 5% CO2/95% air atmosphere, and the medium was renewed every three days.
The expanded progenitor cells (5×105) were washed twice, re-suspended in PBS, and stained with phycoerythrin (PE)-conjugated anti-CD235A (glycophorin) and fluorescein isothiocyanate (FITC)-conjugated anti-human-CD71 (transferrin receptor antibodies). The analysis of the harvested cells was performed on a Beckman-Coulter EPICS XL-MCLs flow cytometer using Beckman-Coulter System II analysis software14; the majority of cells used in the experiments belonged to R4 fraction (early and late basophilic erythroblasts; see Figure 1A).
Figure 1.
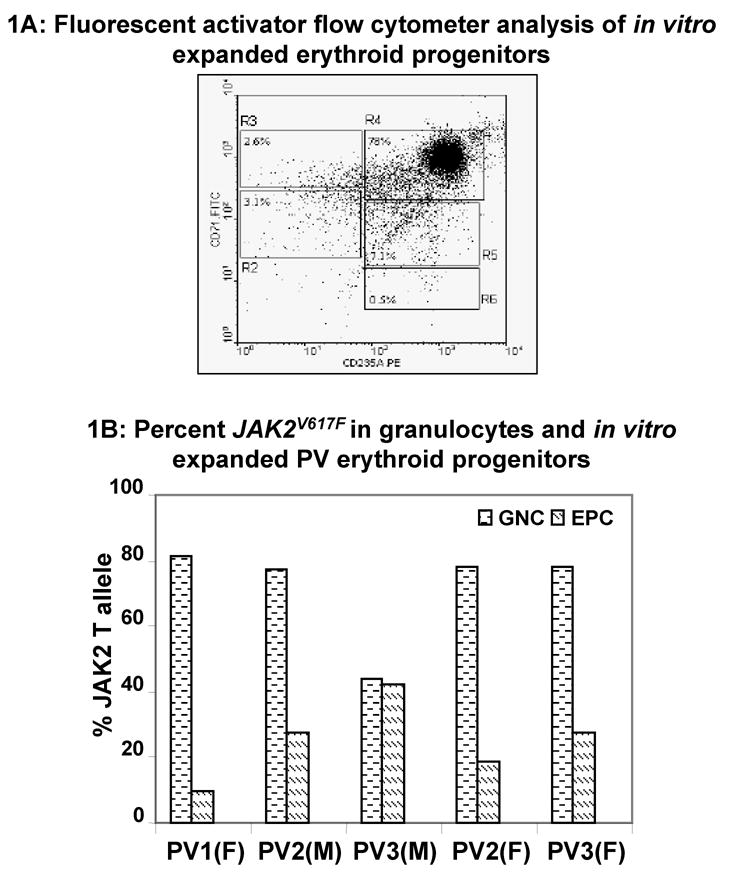
(A) Fluorescent activator flow cytometer analysis of in vitro expanded erythroid progenitors: Mononuclear cells were isolated from PV patients and expanded as described in the Methods. About 5 × 105 cells were stained with PE-conjugated anti-CD235A antibodies (glycophorin) and FITC-conjugated anti-human-CD71 antibodies (transferrin receptor). Regions R2 to R6 are defined by characteristic staining pattern of erythroid cells. These are primitive progenitor cells plus mature BFU-Es and CFU-Es in R2 (CD71med CD235Alow), pro-erythroblasts and early basophilic erythroblasts in R3 (CD71high CD235Alow), early and late basophilic erythroblasts in R4 (CD71high CD235Ahigh), polychromatophilic and orthochromatophilic erythroblasts in R5 (CD71med CD235Ahigh), and late orthochromatophilic erythroblasts and reticulocytes in R6 (CD71low CD235Ahigh). (B) Percent JAK2V617F in granulocytes and in vitro expanded erythroid progenitors: Granulocytes (GNC) were isolated from peripheral blood of PV patients. JAK2V617F was measured in genomic DNA of granulocytes as well as in vitro expanded erythroid progenitors. M and F represent male and female patients, respectively.
Erythropoietin depletion during expansion
Expansion of progenitor cells from MNC of five PV patients was begun as described above. At the end of the first step, cells were divided in two groups. Half the cells were grown in S2 medium without any erythropoietin and the remaining half were grown in the S2 medium containing 3units/ml of erythropoietin. At the end of S2 step of expansion, both the groups were grown separately in complete S3 medium.
Flow cytometric separation of glycophorin positive (CD235+) cells
Mononuclear cells from peripheral blood of PV patients were purified using standard procedures3. About 3×107 cells were stained with PE labeled CD235+ antibody as described (expansion protocol). Cells were analyzed using a Beckman-Coulter cell sorter. The cells that were not stained with PE-anti CD235+ antibodies served as negative control (panel AI of Figure 21), and the sorted CD235+ cells were used to quantitate the total JAK2 T-allele from genomic DNA.
In vitro proliferation of erythroid progenitors
About 1×106 mononuclear cells from the peripheral blood of controls and PV patients were used for in vitro expansion as described. At the end of S3 step, the cells were counted using Beckman automated Z22 coulter counter, cellular proliferation was normalized and expressed as the fold increase in cell number with reference to 106 mononuclear cells.
In vitro generation of dendritic cells
Dendritic cells (DC) were generated by CD14 isolation as previously described15. Briefly, DCs were cultured in CellGenix media with 800 units/ml GM-CSF and 1,000units/ml IL-4. On day 3, IL-4 and GM-CSF were replenished, and on day 5, a maturation cocktail was added containing a final concentration of 10ng/ml IL-1β, 100ng/ml IL-6, 10ng/ml TNF-α, 1 μg/ml PGE2, 800units/ml GM-CSF and 1,000units/ml IL-4. On day 7, DCs were harvested and used as indicated for JAK2V617F, clonality and flow cytometry analysis (not shown).
Quantitation of the JAK2 T-allele
The percent of JAK2 T allele in DNA (isolated using DNAzol extraction kit) was determined in granulocytes, CD235+ cells, in vitro expanded erythroid, and DC using quantitative Real Time allele-specific PCR (Applied Biosystems, Foster City CA) as described16. Each 25μL reaction aliquot consisted of 1× TaqMan Universal PCR master mix, 300nM JAK2 universal forward (gJAK2-F 5′-TTATGGACAACAGTCAAACAACAAT-3′) and allele-specific reverse primers (G-gJAK2-R 5′-TTTACTTACTCTCGTCTCCACAGtC-3′, and T-gJAK2-R 5′-TTTACTTACTCTCGTCTCCACAGtA-3′), 125nM FAM labeled JAK2 MGB probe (FAM-AS-JAK2-MGB 5′-6FAM - CTTGCTCATCATACTTGC – MGBNFQ-3′) and 20ng purified genomic DNA. Temperature cycling consisted of 50 cycles of 95°C for 15sec, and 60°C for 1min following enzyme activation (95°C for 10min). Allele-specific primers were designed using the software program Oligo 6.7 (Molecular Biology Insights, Inc., USA) and synthesized by IDT (Integrated DNA Technologies, USA). The 3′ terminal sequence of the reverse primer (G or T)-gJAK2-R was selected for specific amplification of the mutant allele. Allelic discrimination was enhanced by introducing an artificial mismatch (t:t lower case on primer sequence) in the -1 position starting from the 3′ end of the primer. An additional modified LNA base was placed at the -2 position (G bold and underlined on primer sequence) to increase stability, specificity, and improve consistency during PCR amplification. The quantitative data were verified by pyrosequencing17 with excellent correlation and reproducibility (data not shown); JAK2V617F allele frequency below 0.1% was considered negative.
Clonality assay
Total RNA from granulocytes, in vitro expanded erythroid progenitors and DCs were purified using Trizol reagent. Clonality analysis utilizing BTK, FHL1 and MPP1 exonic polymorphisms was performed following the established procedure18.
Cell lysates and Western blot analysis
Cellular lysates from in vitro expanded erythroid progenitors or from the granulocytes were prepared in the presence of protease and phosphatase inhibitors (50μM sodium orthovanadate and 100μM sodium fluoride) in NP40 lysis buffer. Protein was estimated using Bradford reagent. SDS PAGE and western blot analysis was performed as described19.
Results
In vitro expansion of PV erythroid progenitors causes decrease of JAK2V617F
The three-step expansion procedure for erythroid progenitors yielded cells that were predominantly late basophilic erythroblasts (panel R4 in Figure 1A). These erythroid progenitors from PV patients were more sensitive to imatinib compared to mouse reporter cells expressing human JAK2V617F mutant protein11 (complete data in Gaikwad et al. revised manuscript under review in Exp. Hematology). In order to clarify the molecular mechanism of imatinib mediated growth inhibition in native PV expanded erythroid progenitors, we examined the frequency of JAK2V617F during expansion. To our surprise, the frequency of JAK2V617F in PV erythroid progenitors was decreased in four out of five PV patients tested compared to the JAK2V617F in clonal granulocytes from the same patient (Figure 1B). This finding was subsequently confirmed in a total of 14 patients. JAK2V617F in granulocytes and expanded erythroid progenitors from three healthy controls was not detectable.
Percent JAK2V617F in PV expanded erythroid progenitors and in circulating CD235+ cells
The estimated decrease in JAK2V617F in PV erythroid progenitors was based on the percent JAK2V617F in the clonal granulocytes from the same patient. Examination of JAK2V617F in CD235+ circulating erythroid precursor cells from three PV patients was also examined to further confirm the observed decrease of JAK2V617F during in vitro expansion of PV erythroid progenitors. In vitro expanded erythroid progenitors showed 20–80% decrease of the JAK2V617F allele in comparison to circulating CD235+ cells isolated prior to in vitro expansion (Figure 2B). This result was in agreement with the observed decrease of JAK2V617F percent allele when compared to the granulocyte JAK2V617F (Table 1) for individual patients.
Figure 2.
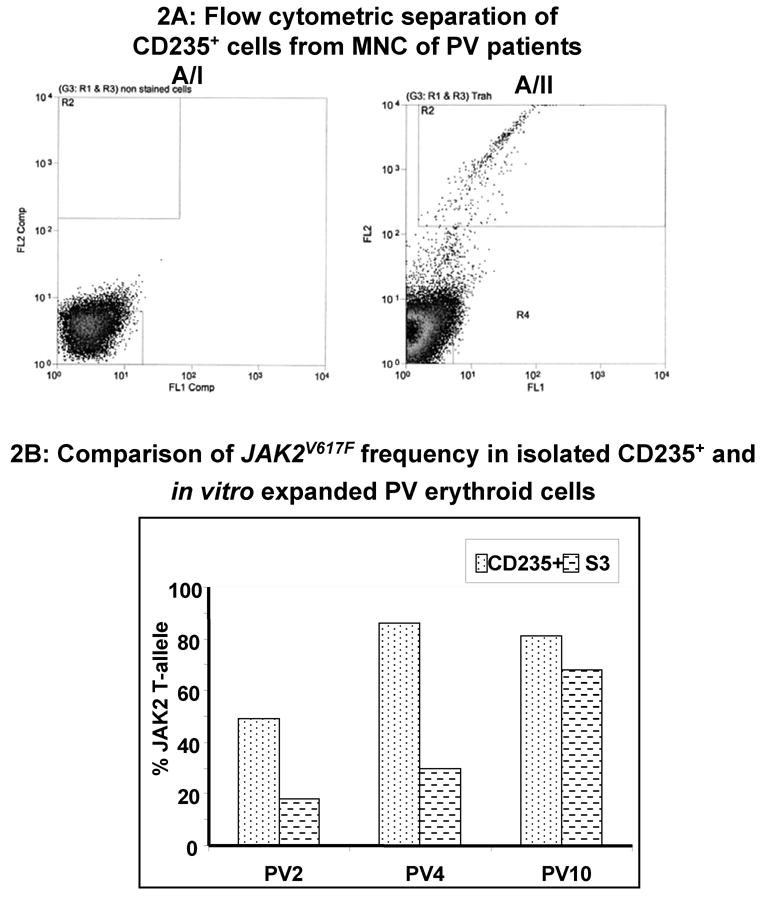
A. Flow cytometric separation of glycophorin positive cells from MNC of PV patients: Mononuclear cells from peripheral blood of PV patients were either unstained (A/I) or immuno-stained with anti-human CD235a antibodies (A/II). Flow cytometric sorting was performed as described in the methods. Cells in the R2 region (CD235a+) were collected. B: JAK2V617F in glycophorin positive and PV erythroid expanded cells: Genomic DNA from CD235a+ was used to measure the JAK2V617F (Gly+) to compare with the JAK2V617F in corresponding in vitro expanded erythroid progenitors (S3) from three PV patients.
Table 1.
Frequency of JAK2V617F during in vitro expansion of PV erythroid progenitors: Genomic DNA was isolated from cells from each of the three steps during in vitro expansion of erythroid progenitors. JAK2V617F was quantitated in granulocytes as well as the erythroid progenitors as described.
| Patients | T-status | GNC | S1 | S2 | S3 | % Dec |
|---|---|---|---|---|---|---|
| 1 | G/T | 65 | 9 | 15 | 9 | 86 |
| 2 | T/T | 97 | 19 | 13 | 18 | 81 |
| 3 | G/T | 52 | 5 | 13 | 13 | 75 |
| 4 | T/T | 97 | 41 | 29 | 30 | 70 |
| 5 | T/T | 79 | 36 | 31 | 24 | 70 |
| 6 | G/T | 28 | 7 | 15 | 11 | 61 |
| 7 | T/T | 83 | 36 | 43 | 39 | 53 |
| 8 | T/T | 89 | 40 | 61 | 52 | 42 |
| 9 | T/T | 81 | 42 | 82 | 66 | 20 |
| 10 | T/T | 84 | 48 | 66 | 68 | 19 |
| 11 | G/T | 47 | 21 | 31 | 41 | 13 |
| 12 | T/T | 86 | 70 | 74 | 79 | 8 |
| 13 | T/T | 96 | 90 | 93 | 91 | 5 |
| 14* | G/T | 37 | 19 | 37 | 37 | 0 |
| 15** | G/T | 50 | 47 | 44 | 50 | 0 |
(Patient #14 is a five-year old female PV patient).
(Patient #15 has idiopathic myelofibrosis). Percent decrease in JAK2V617F in the terminal step of erythroid expansion is calculated relative to the JAK2V617F in the corresponding granulocytes (100%).
JAK2V617F determination during three-step in vitro expansion
As expansion was performed in three steps, we decided to investigate if the loss of JAK2V617F was confined to a specific stage. Analysis of JAK2V617F during the three steps of expansion from 14 PV patients and one patient with myelofibrosis (Table 1, patient #15) was performed. A decrease ranging from 13 to 86% in JAK2V617F was observed at the terminal S3 step of expansion compared to the frequency of JAK2V617F in their respective granulocytes in PV patients #1 – #11. A less significant reduction (5–13%) was observed in PV patients #12– #14. No change in the frequency of JAK2V617F during in vitro expansion was observed in one atypical five- year old PV child (note, PV is very rare in children). Lastly, a PV patient (#15) who developed myelofibrosis also showed no change in the frequency of JAK2V617F in in vitro expanded erythroid cells in comparison to his granulocytes.
In vitro expanded PV erythroid progenitors become polyclonal
Since PV is a clonal disorder, we examined whether the in vitro expanded erythroid progenitors remained clonal or if the dormant non-clonal hematopoietic cells were preferentially expanded along with those belonging to PV clone. While granulocytes from all five female PV patients were clonal, the in vitro expanded erythroid progenitors from all of studied female PV subjects were polyclonal (Figure 3).
Figure 3.
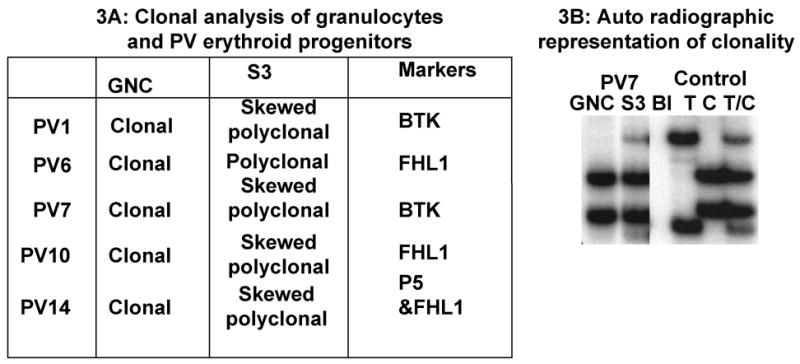
Clonal analysis of granulocytes and PV erythroid progenitors after in vitro expansion: (A) MNC and granulocytes from peripheral blood of five female PV patients was isolated. Expansion of the erythroid progenitors from mononuclear cells was performed as described earlier. Assays for clonality were performed in granulocytes (GNC) and the in vitro expanded erythroid progenitors (S3) using the markers as described17. Skewed polyclonal: Ambiguous or unclear distinction of clonality due to mixed proportion of clonal and polyclonal cells(B) Auto radiographic representation of the clonality analysis. Total RNA was prepared using Trizol reagent from the granulocytes (GNC) isolated from peripheral blood of female PV patients and from the in vitro expanded erythroid cells (S3). The T, C, and TC alleles in the control panel are representative of the standard exonic polymorphism BTK marker described previously17
Apoptosis and proliferation of erythroid progenitors
JAK2V617F mutation in PV causes constitutive activation of the JAK2/STAT5 pathway4 that suppresses apoptosis by STAT5 mediated Bclxl protein expression19. However, we found no significant change in total Bclxl protein between in vitro expanded PV and normal erythroid cells (Figure 4A, panel 1). Furthermore, no significant change was observed in the overall levels of activated/cleaved caspase 3, albeitPV6 that showed moderately elevated levels of cleaved caspase3 compared to controls (Figure 4A, panel 2), suggesting near normal apoptotic regulation in PV erythroid cells during in vitro expansion. In addition, FACS analysis of in vitro expanded erythroid cells stained with propidium iodide/annexin V revealed a comparable percentage of cells (11% and 15%) undergoing apoptosis of normal and PV cells, respectively (data not shown). In contrast, the growth rate of PV erythroid progenitors was ~two-fold enhanced (Figure 4B and C, P<0.05) compared to erythroid progenitors from controls. The rate of proliferation seemed independent of the percent JAK2V617F present in the PV erythroid progenitors at the end of expansion process (S3, Figure 4B).
Figure 4.

Apoptosis and proliferation of in vitro expanded erythroid progenitors: (A) Expansion of the erythroid progenitors from control and PV mononuclear cells was performed as described in methods. Whole cell lysates resolved on SDS12%PAGE were probed for Bclxl, caspase3, and β-actin proteins of control (C) and PV cell lysates. Position of the molecular weight markers is shown on the left. (B) Proliferation of erythroid progenitors was assessed from the total number of cells at the end of S3 step during the in vitro expansion of control and PV erythroid progenitors. Fold increase in the number of cells was determined from the initial total number of MNCs used for expansion. Values from five normal and six PV patients were used to analyze the rate of proliferation. P value was determined using students t-test. *P<0.05. The JAK2V617F allelic frequency of the PV cells at the end of expansion is shown on top.
Frequency of JAK2V617F and clonality analysis of dendritic cells
DCs are potent antigen presenting cells used for cell-based immuno-therapeutic approaches for some malignant hematological disorders. In vitro monocyte derived mature DC from seven PV patients had a phenotype comparable to healthy donors i.e. lineage negative, but HLA-DR and CD83 positive. Monocyte derived mature DC of PV patients showed a decrease in the frequency of JAK2V617F compared to the corresponding granulocytic JAK2V617F (Figure 5A). Clonality analysis of DC from informative female patients revealed that 2 out of 3 DC samples were polyclonal (Figure 5B).
Figure 5.
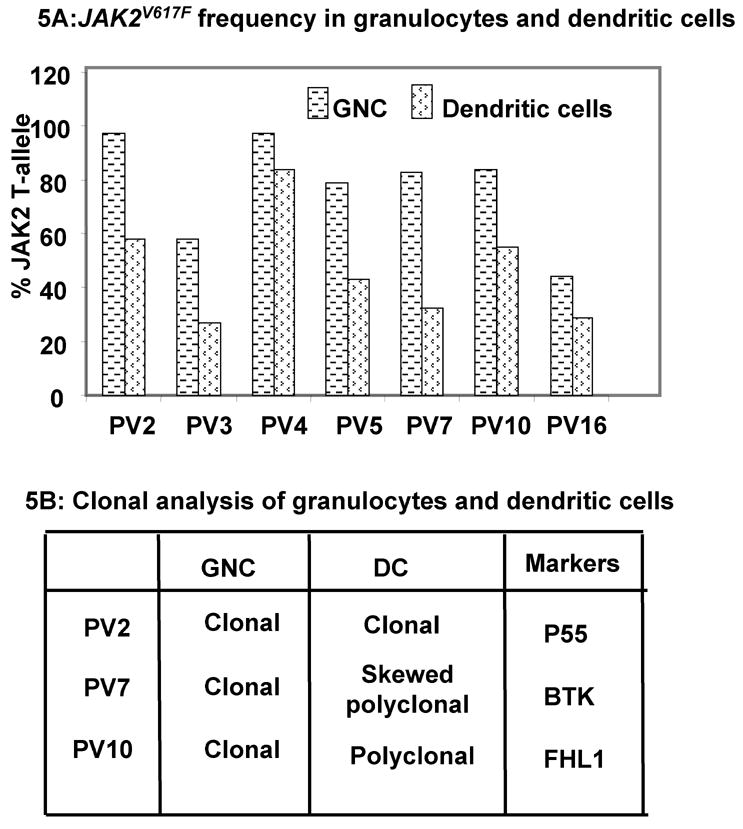
The frequency of JAK2V617F and clonal analysis of dendritic cells: (A) JAK2V617F frequency in granulocytes and dendritic cells was measured as described in methods. (B) Clonal analysis of dendritic cells from three female patients was performed17.
Effect of erythropoietin on JAK2V617F
The hallmark of PV erythroid progenitor cells is erythropoietin hypersensitivity/independence. Erythropoietin exerts its critical effect, early during erythroid differentiation20. Expansion of PV erythroid cells from PV#10–14, (Table 1) in the absence of erythropoietin during the S2 step of in vitro expansion caused a marginal (15-%) increase in the JAK2V617F frequency during the S3 step of expansion (Figure 6: lanes 3 and 4, P<0.05).
Figure 6.
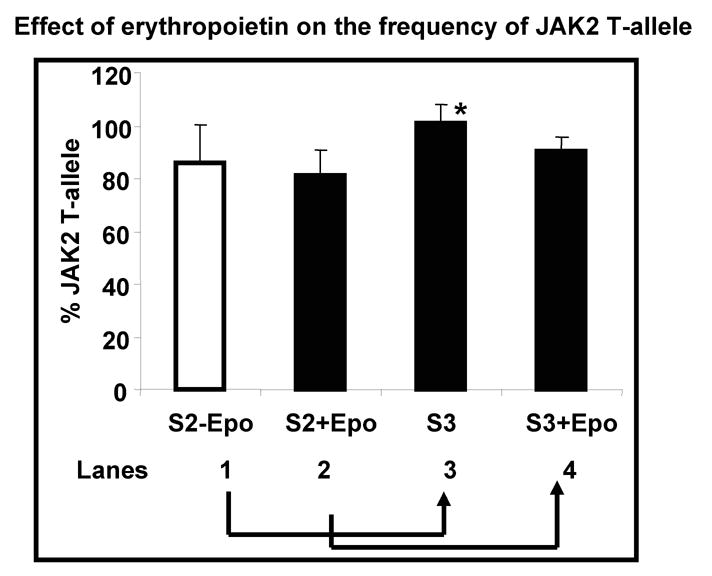
Effect of Epo on JAK2V617F frequency: MNC from five PV patients (PV#10–14) were used for the expansion of erythroid progenitors as described earlier. At the end of S1 step, the cells were divided into two groups. Half the cells were grown in S2 medium without any Epo (S2-Epo: lane1) while the other half was grown in S2 containing 3units/ml of Epo (S2+Epo: lane2). At the end of S2 step of erythroid expansion, both the groups were grown separately, in complete S3 medium (cells from S2-Epo are labeled as S3 (lane3) and cells from S2+Epo are labeled as S3+Epo: lane4, respectively). JAK2V617F frequency was measured at the end of expansion and expressed as percent change in their JAK2V617F frequency using the granulocyte JAK2V617F at a value of 100. Significance was determined using the students’ t-test. * P<0.05.
Discussion
PV is caused by one or more somatic genetic events in a single hematopoietic stem cell resulting in its expansion of its progeny that repopulates all or most mature circulating red cells, platelets, and granulocytes. In rare families with PV22 wherein the PV is not present at birth and is acquired along with conversion of polyclonal to clonal hematopoiesis, at least one of the mutations is germline. Several groups reported a novel somatic mutation in the tyrosine kinase, JAK2V617F in polycythemia vera patients4–7, the most important recent advance in our understanding of the molecular basis of PV. The JAK2V617F mutation has been shown to result in constitutive tyrosine phosphorylation leading to Epo hypersensitivity and independence of erythroid progenitors4–7; a seminal characteristic of PV. Further, the JAK2V617F mutation was sufficient to produce an erythrocytosis resembling PV in mice after autologous transplantation with murine marrow cells retrovirally transduced with JAK2V617F. 4 These observations demonstrate that the JAK2V617F mutation plays an important role in the pathogenesis of PV. Further, Tefferi et al23 have reported a time-dependent increase of JAK2V617F mutation in the granulocytes in some PV patients. However, the exact role for JAK2V617F in the genesis of PV and other myeloproliferative disorders remains unclear. Since majority PV patients have only one mutated JAK2 allele, such a mutation would be expected to be “dominant” and override the effect of a normal JAK2 allele. However, the simultaneous presence of mutated and wild-type JAK2 genes during co-transfection assays abrogated the aberrant behavior of the reporter cells, indicating that JAK2V617F is not a dominant gain-of-function mutation4. Recently, we showed BFU-E colonies from PV patients carrying wild-type JAK2, that can grow without Epo and provided evidence that JAK2V617F mutation is not the PV-initiating event16. Moreover, the JAK2V617F mutation is not PV specific as it is found in other disorders without erythrocytosis17 and 3~20% of PV patients lack the JAK2V617F mutation. Therefore, the relevance and understanding of the exact role of JAK2V617F awaits further clarification.
In our experiments with the effect of tyrosine kinase inhibitors on JAK2V617F, we found that the native in vitro expanded erythroid progenitors from PV patients were more sensitive to imatinib than the mouse reporter cells11. These observations led us to investigate the frequency of JAK2V617F in PV progenitors. Decrease in JAK2V617F during in vitro expansion of PV erythroid progenitors was unexpected since JAK2V617F would be predicted to provide proliferative advantage over wild-type progenitor cells. While one can speculate that these differences between in vivo and in vitro conditions may be due to non-physiological cytokine concentrations present in cell culture conditions or a lack of suitable hematopoietic microenvironment that does not correspond to in vivo conditions; these observations await full elucidation. Interestingly, similar observations have also been made with the preferential loss of Bcr-Abl bearing CML cells during ex vivo expansion24.
PV is characterized by clonal proliferation from myeloid circulating cells and erythropoietin independence of erythroid progenitors. Using an X-chromosome based transcriptional clonality assay18 all informative PV females in our study had clonal reticulocytes granulocytes, platelets, and at times B-lymphocytes3, 25. However, 5 females with PV had polyclonal erythroid progenitors after in vitro expansion (Figure 3) associated with a concomitant decrease of JAK2V617F bearing cells (Table 1). Similarly, majority of the in vitro differentiated DC from the monocytic lineage26 were polyclonal and displayed a consistently lower frequency of the JAK2V617F allele compared to circulating clonal granulocytes (Figure 5).
The observed increased cell number (Figure 4B) of PV erythroid progenitors during in vitro expansion can be attained either by inhibition of apoptosis or via enhanced proliferation. As de-regulated Bclxl levels have been implicated in Epo independent survival of PV erythroblasts27, we analyzed the levels of Bclxl protein and found that in vitro expanded normal and PV erythroid progenitors showed comparable levels of Bclxl protein pointing to normal apoptotic regulation of PV erythroid progenitors during in vitro expansion. Moreover, similar rate of apoptosis observed in normal and PV erythroid progenitors supports this conclusion.
Despite a decrease in the frequency of JAK2V617F in PV erythroid progenitors during in vitro expansion, the PV cells nevertheless maintained their ability to proliferate more rapidly (Figures 4B). The high rate of proliferation of PV erythroid progenitors seemed independent of the JAK2V617F allele frequency. We recently studied a PV patient (PV#16) with JAK2V617F allele frequency of 0.08%. We show that his in vitro expanded erythroid cells, had rate of proliferation comparable to PV#8 (who had 52% JAK2V617F). It however remains to see if higher frequency of JAK2V617F (>50%) will further accentuate the proliferative capacity of PV erythroid cells compared to controls.
Recently Ishi et al28 reported that the proportion of hematopoietic cells expressing the JAK2V617F mutation decreases after in vitro differentiation of CD34+ cells isolated from peripheral blood of PV patients. These findings clearly support our observations in spite of different experimental conditions (Figure 4B). It therefore seems likely that these optimal conditions developed for normal progenitors compromise the competitive advantage of JAK2V617F hematopoietic progenitor cells encountered in vivo (PV1–9, Table 1). Amongst the cytokines used, Epo is known to play a key role in erythropoiesis. To examine the role of Epo in JAK2V617F, under stringent conditions, we chose PV #10–14 that show minimal decrease in their JAK2V617F during in vitro expansion (Table 1). Indeed, removal of Epo during the S2 step of expansion (crucial for the early erythroid development21) showed a definite trend to increased JAK2V617F (Figure 6) during the in vitro expansion. Our observations corroborate the recently published report28 and suggest that the acquired JAK2V617F somatic mutation is likely not the sole event leading to PV.
In conclusion, we show that under in vitro conditions we used here, JAK2V617F mutation bearing PV cells are at a proliferative disadvantage to PV cells without the JAK2V617F mutation, in a striking similarity to what was previously reported in CML23. Our data indicates that in many instances the expansion of polyclonal, possibly suppressed normal hematopoietic cells, accounts for the relative loss of JAK2V617F mutation bearing cells.
Acknowledgments
Research support: supported by grants T32 DK60445-03 (AG and RN, JTP PI), R01HL50077-12 (JTP PI) and MSM 0021620806 (KC and JTP) from the Ministry of Education of the Czech Republic. S.G. is the recipient of a Doris Duke Clinical Scientist Development Award. We thank Drs. L. Rice, J. MacArthur and K. Baker from Baylor College of Medicine for providing peripheral blood of PV patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spivak JL. The chronic myeloproliferative disorders: clonality and clinical heterogeneity. Semin Hematol. 2004;41:1–5. doi: 10.1053/j.seminhematol.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 2.Najfeld V, Montella A, Scalise T, et al. Exploring polycythemia vera with fluorescence in situ hybridization: additional cryptic 9p is the most frequent abnormality detected. Br J Haematol. 2002;119:558–566. doi: 10.1046/j.1365-2141.2002.03763.x. [DOI] [PubMed] [Google Scholar]
- 3.Kralovics R, Guan Y, Prchal JT. Acquired uniparental disomy of chromosome 9p is a frequent stem cell defect in polycythemia vera. Exp Hematol. 2002;30:229–236. doi: 10.1016/s0301-472x(01)00789-5. [DOI] [PubMed] [Google Scholar]
- 4.James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signaling causes polycythemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 5.Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera. Cancer Cell. 2005;4:387–392. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Kralovics R, Passamonti F, Buser AS, et al. A gain of function mutation of JAK2 in myeloproloderative disorders. N Engl J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 7.Zhao R, Xing S, Li Z, et al. Identification of an acquired JAK2 mutation in polycythemia vera. J Biol Chem. 2005;280:22788–22792. doi: 10.1074/jbc.C500138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 9.Baselga J, Arribas J. ‘Treating cancer’s kinase ‘addiction’. Nature Medicine. 2004;10(8):786–787. doi: 10.1038/nm0804-786. [DOI] [PubMed] [Google Scholar]
- 10.Wadleigh M, DeAngelo DJ, Griffin JD, Stone RM. After chronic myelogenous leukemia: tyrosine kinase inhibitors in other hematologic malignancies. Blood. 2005;105:22–30. doi: 10.1182/blood-2003-11-3896. [DOI] [PubMed] [Google Scholar]
- 11.Gaikwad A, Verstovsek S, Chang K, Yoon D, Vainchenker W, Prchal JT. Will Imatinib be Useful for Patients with Polycythemia Vera? Blood. 2005;106(11):2601. [Google Scholar]
- 12.Prchal J, Chang K, Jelinek J, et al. In vitro expansion of polycythemia vera progenitors favors expansion of erythroid precursors without JAK2 V617F mutation. Blood. 2005;106(11):3506. [Google Scholar]
- 13.Neildez-Nguyen TM, Wajcman H, Marden MC, et al. Human erythroid cells produced ex vivo at large scale differentiate into red blood cells in vivo. Nature Biotechnol. 2002;20:467–472. doi: 10.1038/nbt0502-467. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Ma S, Gross AW, Lodish HF. Role of Ras signaling in erythroid differentiation of mouse fetal liver cells: functional analysis by a flow cytometry-based novel culture system. Blood. 2003;102:3938–3946. doi: 10.1182/blood-2003-05-1479. [DOI] [PubMed] [Google Scholar]
- 15.Gottschalk S, Edwards OL, Sili U, et al. Generating CTLs against the subdominant Epstein-Barr virus LMP1 antigen for the adoptive immunotherapy of EBV-associated malignancies. Blood. 2003;101:1905–1912. doi: 10.1182/blood-2002-05-1514. [DOI] [PubMed] [Google Scholar]
- 16.Nussenzveig R, Swierczek S, Jelinek J, et al. Polycythemia vera is not initiated by JAK2V617F mutation. Exp Hematol. 2007 doi: 10.1016/j.exphem.2006.11.012. In Press. [DOI] [PubMed] [Google Scholar]
- 17.Jelinek J, Oki Y, Gharibyan V, et al. JAK2 mutation 1849G>T is rare in acute leukemias but can be found in CMML, Philadelphia chromosome–negative CML, and megakaryocytic leukemia. Blood. 2005;106:3370–3373. doi: 10.1182/blood-2005-05-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu E, Jelinek J, Pastore Y, et al. Discrimination of polycythemias and thrombocytoses by novel, simple, accurate clonality assays and comparison with PRV-1 expression and BFU-E response to erythropoietin. Blood. 2003;101:3294–3301. doi: 10.1182/blood-2002-07-2287. [DOI] [PubMed] [Google Scholar]
- 19.Gaikwad A, Poblenz A, Haridas V, Zhang C, Duvic M, Gutterman J. Triterpenoid Electrophiles (Avicins) Suppress Heat Shock Protein-70 and X-Linked Inhibitor of Apoptosis Proteins in Malignant Cells by Activation of Ubiquitin Machinery: Implications for Proapoptotic Activity. Clinical Cancer Res. 2005;11:1953–1962. doi: 10.1158/1078-0432.CCR-04-1704. [DOI] [PubMed] [Google Scholar]
- 20.Battle T, Frank D. The Role of STATs in Apoptosis. Current Molecular Medicine. 2002;2:381–392. doi: 10.2174/1566524023362456. [DOI] [PubMed] [Google Scholar]
- 21.Fisher MJ, Prchal JF, Prchal JT, D’Andrea AD. Anti-erythropoietin (EPO) receptor monoclonal antibodies distinguish EPO-dependent and EPO-independent erythroid progenitors in polycythemia vera. Blood. 1994;84(6):198201991. [PubMed] [Google Scholar]
- 22.Kralovics R, Stockton DW, Prchal JT. Clonal hematopoiesis in familial polycythemia vera suggests the involvement of multiple mutational events in the early pathogenesis of the disease. Blood. 2003;102(10):3793–3796. doi: 10.1182/blood-2003-03-0885. [DOI] [PubMed] [Google Scholar]
- 23.Tefferi A, Lasho TL, Gilliland G. JAK2 mutations in myeloproliferative disorders. N Engl J Med. 2005;353:1416–1417. doi: 10.1056/NEJMc051878. [DOI] [PubMed] [Google Scholar]
- 24.Petzer A, Eaves C, Barnett M, Eaves A. Selective Expansion of Primitive Normal Hematopoietic Cells in Cytokine-Supplemented Cultures of Purified Cells from Patients with Chronic Myeloid Leukemia. Blood. 1997;90:64–69. [PubMed] [Google Scholar]
- 25.Prchal JT. Polycythemia vera and other primary polycythemias. Curr Opin Hematol. 2005;12:112–116. doi: 10.1097/01.moh.0000154029.05396.d2. [DOI] [PubMed] [Google Scholar]
- 26.Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin in Immunol. 1997;9:10–16. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 27.Silva M, Richard C, Benito A, Sanz C, Olalla I, Fernandez-Luna JL. Expression of Bcl-x in erythroid precursors from patients with polycythemia vera. N Engl J Med. 1998;338(9):564–571. doi: 10.1056/NEJM199802263380902. [DOI] [PubMed] [Google Scholar]
- 28.Ishii T, Bruno E, Hoffman R, Xu M. Involvement of various hematopoietic-cell lineages by the JAK2V617F mutation in polycythemia vera. Blood. 2006;108:3128–3134. doi: 10.1182/blood-2006-04-017392. [DOI] [PubMed] [Google Scholar]


