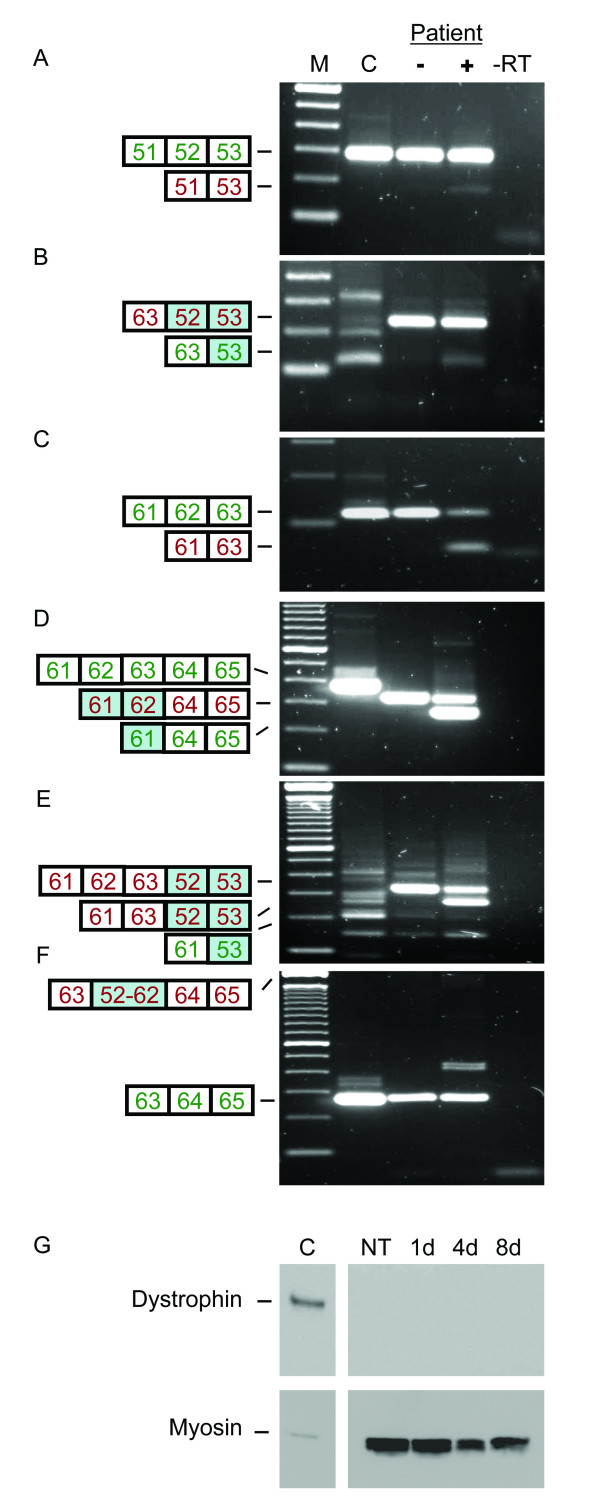
Figure 5.
Exon skipping in myotube cultures from a patient with an exon 52–62 duplication. A-F. RT-PCR analysis. After treatment (+) specific skipping of the original and the duplicated exons 52 and 62 was induced in patient myotubes, while these skips were not found before treatment (-) (A-D). Since the duplicated exons are located between exon 63 and 64, the patient appears to have an exon 63 deletion using primers flanking this exon (D). Skipping of the original exons 62, 63, and the duplicated exon 52 could be detected at low levels in untreated myotube cultures (E). After AON treatment, these levels increased and, in addition, single exon 62 skipping was induced. Primers specific for the duplication (B and E) generated only non specific fragments in the control sample, as confirmed by sequencing analysis (C). Using primers flanking the duplication (F) normally spliced transcripts were detected both in untreated and treated patient RNA, albeit at lower levels than the control. The expected fragment of ~1900 bp containing the duplication was not observed (upper marker band is 2 kb). In-frame and out-of-frame transcripts are shown in green and red, respectively. Duplicated exons are shaded in blue. M is 100 bp DNA size marker, -RT is negative control. G. Western blot analysis. No dystrophin could be detected in protein isolated from untreated (NT) myotubes, or from myotubes isolated 1, 4 and 8 days after AON treatment. A clear dystrophin signal (dy4) is present in protein isolated from unaffected control myotube cultures (C) (diluted 1:15 to prevent overexposure). Myosin (MF20) signals could be detected in both treated and untreated patient samples, indicating that the differentiation stage of the myotubes was sufficient to allow dystrophin synthesis.