Abstract
The present study characterized the single-dose pharmacokinetics of daptomycin dosed as 4 mg/kg of total body weight (TBW) in seven morbidly obese and seven age-, sex-, race-, and serum creatinine-matched healthy subjects. The glomerular filtration rate (GFR) was measured for both groups following a single bolus injection of [125I]sodium iothalamate. Noncompartmental analysis was used to determine the pharmacokinetic parameters, and these values were normalized against TBW, ideal body weight (IBW), and fat-free weight (FFW) for comparison of the two groups. All subjects enrolled in this study were female, and the mean (±standard deviation) body mass index was 46.2 ± 5.5 kg/m2 or 21.8 ± 1.9 kg/m2 for the morbidly obese or normal-weight group, respectively. The maximum plasma concentration and area under the concentration-time curve from dosing to 24 h were approximately 60% higher (P < 0.05) in the morbidly obese group than in the normal-weight group, and these were a function of the higher total dose received in the morbidly obese group. No differences in daptomycin volume of distribution (V), total clearance, renal clearance, or protein binding were noted between the two groups. Of TBW, FFW, or IBW, TBW provided the best correlation to V. In contrast, TBW overestimated GFR through creatinine clearance calculations using the Cockcroft-Gault equation. Use of IBW in the Cockcroft-Gault equation or use of the four-variable modification of diet in renal disease equation best estimated GFR in morbidly obese subjects. Further studies of daptomycin pharmacokinetics in morbidly obese patients with acute bacterial infections and impaired renal function are necessary to better predict appropriate dosage intervals.
The prevalence of obesity in the United States has reached epidemic proportions. According to the National Center for Health Statistics, 30% of the U.S. population is estimated to be obese and 5% is estimated to be morbidly obese (13). These projections are cause for concern given that obesity is considered to be an independent risk factor for morbidity and mortality among surgical and critically ill patients (1, 18, 19). This emerging population poses a significant challenge to clinicians when considering antimicrobial dosing for prophylaxis and treatment of surgical site infections (12, 15).
Daptomycin is a novel lipopeptide antibiotic that is active against common resistant gram-positive bacteria, such as methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus spp. (CUBICIN [daptomycin for injection] package insert; Cubist Pharmaceuticals, Inc., Lexington, MA). Daptomycin is typically dosed as 4 to 6 mg/kg of total body weight (TBW) as a once-daily infusion, and the interval is adjusted based on renal function (CUBICIN package insert; Cubist Pharmaceuticals, Inc., Lexington, MA). According to the daptomycin package labeling, TBW is deemed the appropriate dosing weight for morbidly obese subjects (CUBICIN package insert; Cubist Pharmaceuticals, Inc., Lexington, MA). This conclusion was based on a study of six morbidly obese subjects which demonstrated that the clearance (CL) and the area under the concentration-time curve (AUC) of daptomycin increased by 46% and 31%, respectively, compared to levels for nonobese controls (11). This increased CL of daptomycin was consistent with the population studied, given that daptomycin CL is directly proportional to the glomerular filtration rate (GFR) and that higher GFRs are noted for obese individuals than for normal-weight controls (4, 9).
Estimation of GFR is performed clinically by measurements of creatinine clearance (CLCR) but is often overestimated due to the influence of active renal tubular secretion of creatinine (24). In addition, the most common equation used to calculate CLCR (Cockcroft-Gault) can overestimate GFR because it is a weight-based equation (5, 23). In an effort to improve the accuracy of GFR estimates, the National Kidney Foundation has recently recommended the use of the four-variable modification of diet in renal disease (MDRD) equation (16). However, the MDRD equation was derived from patients with renal dysfunction and did not include morbidly obese subjects. Despite these inherent limitations of estimating GFR, a previous evaluation of daptomycin pharmacokinetics in morbidly obese individuals did not match normal-weight subjects for renal function by using stringent criteria (11). The current study compared the pharmacokinetics of daptomycin dosed on TBW in morbidly obese patients to the pharmacokinetics for age-, sex-, race-, and serum creatinine-matched, normal-weight, healthy subjects. We also determined GFR by using [125I]sodium iothalamate to define renal function and estimated fat-free weight (FFW) through bioelectric impedance analysis (BIA) to characterize daptomycin plasma pharmacokinetic parameters.
MATERIALS AND METHODS
Study protocol.
The study protocol was approved by the Human Research Review Committee and the Human Uses Subcommittee for Radiation Safety at the University of New Mexico Health Sciences Center (UNMHSC). The study protocol was also approved by the Institutional Review Board at the University of Illinois at Chicago. Healthy subjects were informed in detail about the purpose, procedures, risks, and lack of direct benefits from participating in this research. All subjects provided signed informed consent prior to inclusion into the study. The study was performed at the UNMHSC General Clinical Research Unit (GCRC).
Inclusion criteria.
Subjects fulfilling the following criteria were eligible to participate: (i) males or females, 18 to 50 years of age; (ii) nonsmoking subjects, defined as no smoking of cigarettes within the last 90 days; (iii) subjects with a body mass index (BMI) of >40 kg/m2, with a BMI between 18 and 25 kg/m2 in matched normal-weight volunteers; (iv) subjects with a TBW of >200 lbs (obese subjects only); (v) subjects with serum creatinine of <1.4 mg/dl; and (vi) female subjects of childbearing potential either surgically sterilized or using an effective method of contraception.
Exclusion criteria.
Subjects were not eligible if any of the following criteria were met: (i) history of significant hypersensitivity reaction or intolerance to daptomycin or iodine; (ii) history of significant cardiac, neurological, thyroid, muscular, or immune disorder; (iii) absolute neutrophil count of <1 × 109/liter; (iv) transaminase (aspartate aminotransferase or alanine aminotransferase) of >2.5× the upper limit of normal; (iv) estimated CLCR of <50 ml/min/1.73 m2 (MDRD equation); (v) abnormal serum electrolyte results; (vi) abnormal creatine phosphokinase (CPK) concentrations; (vii) positive urine pregnancy test; (viii) abnormal electrocardiogram; (ix) intolerance to venipuncture and multiple blood draws; (x) intolerance to 1.5- to 2.0-liter infusions of normal saline (for GFR determination); (xi) unwillingness to abstain from alcohol, cigarette smoking, and caffeine intake during study period (5 days); (xii) clinically significant abnormal physical examination, defined as a physical finding requiring further workup or management with a pharmacological or nonpharmacological intervention; and (xiii) inability to follow instructions, in the opinion of the investigator.
Subject matching criteria.
Normal-weight, healthy subjects identified through the recruitment process were matched to enrolled morbidly obese subjects by age, sex, race, and serum creatinine. The ages of the adult, healthy, normal-weight subjects were ±5 years those of the matched morbidly obese subjects and the serum creatinine values ±0.1 mg/dl those of the matched morbidly obese subjects.
Determination of GFR.
Subjects received a 2-day supply of Lugol's solution (4.5 to 5.5% iodine) that was administered as three drops by mouth thrice daily, mixed in orange juice. The purpose of this procedure was to reduce radioactive iodine (125I) uptake by the thyroid. The subjects were admitted at ∼9 a.m. on the third day of the study for determination of GFR by use of Cohen's method (6). A peripheral 18- to 20-gauge intravascular catheter was inserted into the antecubital vein of the nondominant arm. A two-port 3-way stopcock was used to access blood samples during the study. A 0.9% sodium chloride infusion was initiated at 500 ml per hour for 4 h. After an hour of infusion, the subject was asked to empty his or her bladder into a urine collection jug labeled “urine control.” A 20- to 30-μCi dose of [125I]sodium iothalamate (Cypros Pharmaceuticals, Carlsbad, CA) was injected intravenously. After approximately 30 to 60 min, the subject was asked to empty his or her bladder into a urine collection jug labeled “urine discard.” A 5-ml blood sample was immediately collected in a heparinized tube labeled “plasma no. 1.” After an additional 30 to 60 min, the subject again voided into a urine collection jug labeled “urine no. 1.” Another blood sample was collected and labeled “plasma no. 2.” The last urine sample was collected 30 to 60 min later and labeled “urine no. 2.” A final blood sample was collected and labeled “plasma no. 3.” A 0.45% sodium chloride infusion was run through the peripheral line at 40 to 50 ml per hour to maintain patency. Subjects were not allowed to eat or drink (except water) after 10 p.m. on day 3 in order to complete BIA in the morning.
BIA.
Subjects emptied their bladders prior to BIA and were required to remove any metallic jewelry. The subject's height and weight were recorded, and measurements of the circumferences of the hip and waist taken. BIA was performed using a Quantum system (RJL Systems, Clinton, MI), which included a rapid (5-min) process. The subject was asked to lie horizontally on the examination table. Electrolyte gel was applied and spot electrodes placed on bare surfaces of the right hand and foot. A 50-kHz current was applied and measurements of the resistance and reactance noted. The procedure was repeated using the left hand and foot. Determination of fat and lean weight was performed using Comprehensive Body Composition software (Human Kinetics, Champaign, IL, 1997). Equations specific for determination of fat weight in morbidly obese patients were used (14).
Daptomycin sampling.
Upon completion of BIA, a blood sample was collected (predose) and subjects received a single 4-mg/kg daptomycin infusion over 30 min that was dosed on TBW. Blood samples were collected in lithium heparin tubes at 0.5 (end of infusion), 1.0, 1.5, 2.0, 4.0, 8.0, 12.0, and 24 h from the start of infusion. In addition, blood samples were collected in serum separator tubes at 0.5, 2, and 8 h for determination of daptomycin protein binding. A 24-h urine collection was performed by asking the subject to void prior to daptomycin infusion and then collecting urine for 24 h after infusion of daptomycin to determine the CLR of daptomycin.
Safety assessment and management.
Safety assessments were performed on each of the study days. All observed and subjected adverse reactions were noted on the study records, regardless of association with study drugs. The records indicated the specific time of onset, severity, treatment, outcome, and duration of each episode. No additional medications were allowed during the study period, except acetaminophen for symptomatic relief of headaches. Aspirin or nonsteroidal anti-inflammatory agents such as ibuprofen, naproxen, and ketoprofen were not permitted during the study.
Sample analysis.
The blood samples collected during the daptomycin sampling phase were centrifuged at 1,500 × g for 10 min, and the harvested plasma or serum samples were stored at −80°C until analysis. Plasma and urine samples collected during the GFR determination phase were analyzed using a well scintillation detector with a single-channel pulse height analyzer. The time setting for radioactivity determination was 2 min for urine samples or 20 min for plasma samples. Similarly, 1-ml aliquots of the 24-h urine collection taken during the daptomycin phase were stored frozen. Plasma, serum, and urine samples were shipped as a batch to the Center for Anti-Infective Research and Development at Hartford Hospital (Hartford, CT) for analysis of daptomycin concentrations by use of a validated high-performance liquid chromatography method described previously (7). Briefly, reverse-phase high-performance liquid chromatography was used with UV detection at 214 nm over a range of 2 to 100 μg/ml. The interrun and intrarun coefficients of variation ranged from 2.57% to 6.20% for the three matrices (plasma, serum, and urine).
Daptomycin serum protein binding was measured by equilibrium dialysis, solid-phase extraction, and liquid chromatography-mass spectrophotometry, with a linearity range of 1 to 250 μg/ml as previously described (10). Analysis of daptomycin protein binding was performed at Cubist Pharmaceuticals, Inc. (Lexington, MA). The coefficient of variation for the quality control samples for the specified linear range was 3.9 to 7.6%.
Pharmacokinetic analysis.
Plasma and urine daptomycin concentrations were used to determine pharmacokinetic parameters by use of WinNonlin pharmacokinetic software, version 5.01 (Pharsight, Mountain View, CA). The maximum plasma concentration (Cmax) and the minimum (24-h) plasma concentration (C24) were determined directly without interpolation. The apparent elimination rate constant (kel) was determined through regression analysis of the logarithmic linear portion of the concentration-time curve. The linear trapezoidal rule was used to determine the AUC from dosing to 24 h (AUC0-24). The AUC from dosing to infinity (AUC0-∞) was calculated as a function of the sum of AUC0-24 and the residual area after 24 h based on C24/kel. The elimination half-life (t1/2) was calculated based on (ln 2)/kel. Total plasma daptomycin clearance (CLT) was calculated by dividing the dose of daptomycin by AUC0-∞, and daptomycin renal clearance (CLR) was calculated based on the total amount of daptomycin recovered in urine over 24 h divided by the plasma daptomycin AUC0-24. The daptomycin volume of distribution (V) during the terminal phase was determined by CLT/kel.
Statistical analysis.
Student's t test (paired) after assessments for equality of variances using Levene's test was used to compare continuous data after eliminating one normal-weight control, who had been matched to the non-evaluable morbidly obese subject. Data deemed nonnormally distributed by the Shapiro-Wilks test were compared by the Wilcoxon signed-rank test between the morbidly obese and control subjects. Fisher's exact test was used to compare nonparametric demographic variables. A P value of <0.05 was defined as statistically significant. Linear regression was used to determine the association of daptomycin CLT and CLR with measured GFR and calculated GFR through the four-variable MDRD equation. Daptomycin CL was also compared to CLCR calculated by the Cockcroft-Gault method including use of TBW, ideal body weight (IBW), and FFW.
RESULTS
Subject demographics.
Forty-nine overweight subjects (18% male) contacted the investigators for participation in the study. Unfortunately, no male subject presented to the UNMHSC GCRC for the screening visit and consequently 10 female, morbidly obese, healthy subjects were screened. One subject withdrew from the study after the screening visit, and an additional subject was excluded secondary to a diagnosis of diabetes mellitus. Consequently, 16 subjects were enrolled and completed the study, with eight subjects in each group (morbidly obese and normal weight). Plasma analysis for daptomycin was indeterminate in one morbidly obese subject, and so data were comparable for seven morbidly obese and seven matched normal-weight subjects.
The demographic characteristics of the seven morbidly obese subjects and the seven matched controls are described in Table 1. All subjects were female, with a mean (±standard deviation [SD]) age of 36.8 ± 11.0 and 29.1 ± 12.0 years for the morbidly obese and normal-weight groups, respectively. Both groups were well matched for age, sex, race, serum creatinine, and serum albumin. The mean BMI of morbidly obese subjects was approximately twice that of the normal-weight group. The estimated FFW was approximately 10 kg higher in the morbidly obese group than in the normal-weight group and was accurately predicted by the IBW equation in the morbidly obese group. Although the mean GFR estimated through [125I]sodium iothalamate CL was 24% higher in the morbidly obese group than in the normal-weight group, the data did not reach a statistically significant level. Use of the Cockcroft-Gault equation with IBW and not TBW provided the most comparable assessment of GFR. The non-weight-based four-variable MDRD equation also provided the most comparable assessment of GFR in both groups.
TABLE 1.
Comparison of patient demographic variables between morbidly obese and normal-weight subjects
| Variable | Value for subject group
|
P value | |
|---|---|---|---|
| Morbidly obese (n = 7) | Normal weight (n = 7) | ||
| Age (yr) | 36.8 ± 11.0 | 29.1 ± 12 | 0.053 |
| Gender (% of subjects) | Female (100) | Female (100) | 1.0 |
| Race (no. [%] of subjects) | 1.0 | ||
| White | 3 (43) | 3 (43) | |
| Hispanic | 3 (43) | 3 (43) | |
| Black | 1 (14) | 1 (14) | |
| BMI (kg/m2) | 46.2 ± 5.5 | 21.8 ± 1.9 | 0.00001a |
| TBW (kg) | 114.3 ± 15.8 | 58.8 ± 6.2 | 0.00001a |
| IBW (kg) | 52.8 ± 6.2 | 55.8 ± 6.1 | 0.46 |
| FFW (kg) | 54.7 ± 5.7 | 44.3 ± 8.5 | 0.006a |
| Serum creatinine (mg/dl) | 0.8 ± 0.2 | 0.8 ± 0.1 | 0.46 |
| Albumin (mg/dl) | 3.9 ± 0.4 | 4.2 ± 0.2 | 0.23 |
| GFR (ml/min) measured by [125I]Na iothalamate | 116.0 ± 45.2 | 93.5 ± 28.8 | 0.36 |
| GFR (ml/min/1.73 m2) estimated by four-variable MDRDb | 100.8 ± 28.7 | 93.0 ± 13.4 | 0.46 |
| CLCR (ml/min) measured by Cockcroft-Gaultc with: | |||
| TBW | 226.9 ± 69.1 | 112.2 ± 19.7 | 0.003a |
| IBWd | 101.0 ± 21.1 | 107.6 ± 24.2 | 0.49 |
| FFWe | 106.7 ± 32.7 | 85.3 ± 21.3 | 0.061 |
Significantly different between the two groups.
MDRD equation to estimate GFR is as follows: 186 × serum creatinine−1.154 × age−0.203 (× 0.742 if female) (× 1.210 if black).
Cockcroft-Gault equation to estimate GFR through the surrogate CLCR is as follows: [(140 − age) × weight (× 0.85 if female)]/(serum creatinine × 72).
IBW is estimated as follows: 45.5 + 2.3 × number of inches over 5 feet.
FFW was determined using BIA.
Daptomycin pharmacokinetics.
A summary of comparative pharmacokinetic parameters is provided in Table 2. Statistical analysis using a paired t test after eliminating one matched normal-weight control, i.e., for seven morbidly obese subjects and seven matched normal-weight controls, did not alter the significance of the reported pharmacokinetic parameters between the groups compared to statistical analysis by an unpaired t test using all evaluable subjects. As expected, a TBW-based dosing (4 mg/kg) approach equated to almost twice the total dose for the morbidly obese patients than for the normal-weight patients. The absolute V and CLT, although higher in the morbidly obese group, were not statistically different from values for the normal-weight controls. Consequently, the approximately 60% higher Cmax and AUC0-24 values in the morbidly obese group were a function of total dose administered (P < 0.001). Total weight-normalized AUC0-24 and Cmax values were not statistically different between groups, confirming that differences noted between groups were a function of total dose administered.
TABLE 2.
Comparison of patient pharmacokinetic variables between morbidly obese and normal-weight subjects
| Variable | Value for subject group
|
P value | |
|---|---|---|---|
| Morbidly obese (n = 7) | Normal weight (n = 7) | ||
| Total dose (mg) | 461 ± 61 | 236 ± 26 | 0.0001a |
| Cmax (mg/liter) | 67.3 ± 12.3 | 42.3 ± 11.9 | 0.029a |
| Cmax (mg/liter/kgTBW) | 0.61 ± 0.16 | 0.72 ± 0.18 | 0.41 |
| C24 (mg/liter) | 6.55 ± 2.31 | 3.39 ± 0.79 | 0.011a |
| AUC0-24 (mg·h/liter) | 494 ± 62 | 307 ± 54 | 0.002a |
| AUC0-24 (mg·h/liter/kgTBW) | 4.41 ± 0.85 | 5.25 ± 0.82 | 0.18 |
| AUC0-∞ (mg·h/liter) | 581 ± 104 | 346 ± 63 | 0.003a |
| AUC0-∞ (mg·h/liter/kgTBW) | 5.17 ± 1.11 | 5.90 ± 0.94 | 0.29 |
| V (liter) | 10.04 ± 2.04 | 7.69 ± 1.05 | 0.066 |
| V (liter/kgTBW) | 0.09 ± 0.01 | 0.13 ± 0.02 | 0.003a |
| V (liter/kgIBWb) | 0.19 ± 0.04 | 0.14 ± 0.02 | 0.033a |
| V (liter/kgFFWc) | 0.18 ± 0.03 | 0.18 ± 0.03 | 0.77 |
| CL (liter/h) | 0.82 ± 0.21 | 0.73 ± 0.14 | 0.34 |
| CL (liter/h/kgTBW) | 0.0071 ± 0.0013 | 0.012 ± 0.0020 | 0.002a |
| CL (liter/h/kgIBW) | 0.016 ± 0.0013 | 0.013 ± 0.0020 | 0.16 |
| CL (liter/h/kgFFW) | 0.015 ± 0.0034 | 0.016 ± 0.0030 | 0.60 |
| CLR (liter/h) | 0.50 ± 0.11 | 0.59 ± 0.28 | 0.38 |
| CLR (liter/h/kgTBW) | 0.0044 ± 0.0010 | 0.0095 ± 0.0036 | 0.003a |
| CLR (liter/h/kgIBW) | 0.0096 ± 0.0027 | 0.0099 ± 0.0034 | 0.79 |
| CLR (liter/h/kgFFW) | 0.0092 ± 0.0024 | 0.013 ± 0.0050 | 0.042 |
| kel (h−1) | 0.08 ± 0.01 | 0.09 ± 0.01 | 0.142 |
| t1/2 (h) | 8.68 ± 1.67 | 7.72 ± 0.76 | 0.134 |
| Protein binding (%) | 88.8 ± 1.6 | 90.0 ± 1.8 | 0.146 |
Significantly different between the two groups.
IBW was estimated as follows: 45.5 + 2.3 × number of inches over 5 feet.
FFW was determined using BIA.
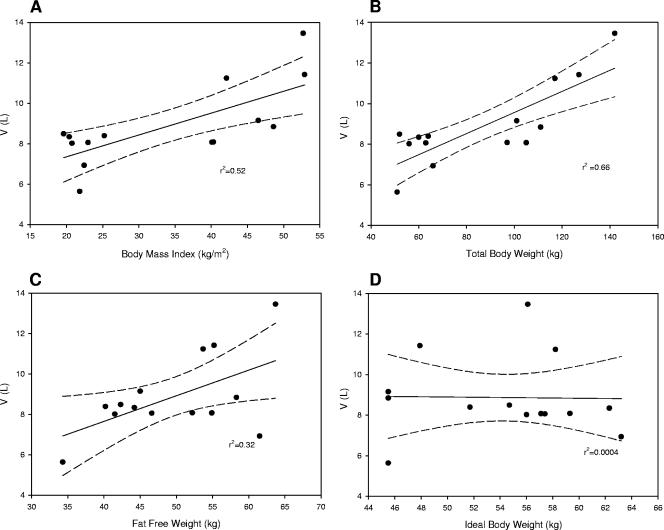
Comparison of V results in relation to TBW, IBW, and FFW revealed that defining V as a function of TBW or IBW characterized morbidly obese patients with values that were statistically significant compared to values for normal-weight controls. In contrast, defining V as a function of FFW provided nearly identical results (V = 0.18 liter/kgFFW) in both groups, implying that fat weight does not contribute proportionately to the distribution of daptomycin. Despite these associations, the relationship of V to weight was best predicted by TBW (r2 = 0.66) and BMI (r2 = 0.52) by linear regression when evaluating both groups together (Fig. 1). The relationship of V to FFW was poorer (r2 = 0.32), and no relationship was revealed when comparing IBW to daptomycin V (r2 = 0.0004). Furthermore, TBW provided the best (r2 = 0.89) estimate of V in morbidly obese subjects when the linear regression-derived function was used, i.e., V = (0.12 × TBW) − 3.86.
FIG. 1.
Relationships of daptomycin V to BMI, TBW, FFW, and IBW.
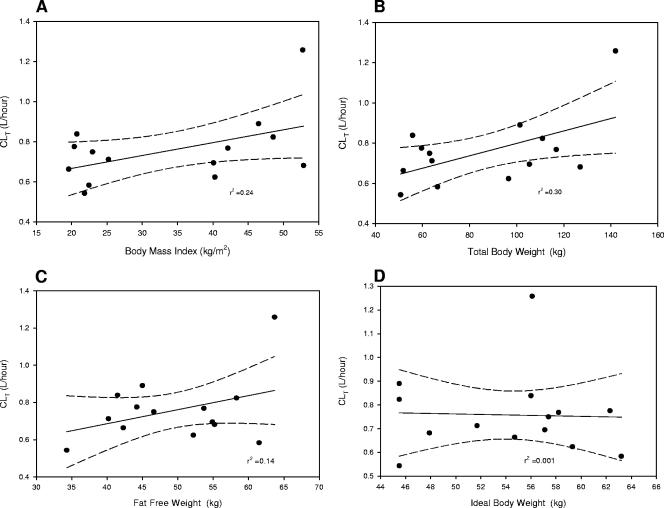
Similarly, CLT as a function of TBW characterized the two groups as statistically different even though the absolute levels of CLT were not significantly different between morbidly obese and normal-weight subjects. Levels of normalization of CLT to either IBW or FFW were not statistically different between the two groups. Again, the relationship of CLT (Fig. 2) was best predicted by TBW (r2 = 0.30) and BMI (r2 = 0.24) compared to FFW (r2 = 0.14) and IBW (r2 = 0.001). No statistically significant differences were noted between the groups for daptomycin CLR, and the mean (±SD) urine daptomycin recovery over 24 h was 43% ± 7% or 53% ± 17% for the morbidly obese or normal-weight group, respectively (P = 0.12). The daptomycin t1/2 was approximately 8 h in both groups, and protein binding levels (90%) were also similar.
FIG. 2.
Relationships of daptomycin CLT to BMI, TBW, FFW, and IBW.
Daptomycin CL and GFR.
A significant (P < 0.01) correlation was noted between daptomycin CLT (liter/h) and measured GFR (r2 = 0.41), GFR estimated using MDRD (r2 = 0.57), and CLCR estimated using the Cockcroft-Gault equation with TBW (r2 = 0.49). Use of the Cockcroft-Gault equation with IBW or FFW did not have a statistically significant (P > 0.1) correlation with daptomycin CLT. Despite this relationship, the Cockcroft-Gault equation using TBW as an estimate of GFR was biased, with a mean (95% confidence interval [95% CI]) bias of 69 (40 to 98) ml/min for the combined groups. In contrast, the MDRD equation provided an unbiased estimate of GFR, with a mean (95% CI) bias of −8 (−22 to 6) ml/min for the combined groups. Similarly, the Cockcroft-Gault equation using IBW as an estimate of GFR was unbiased, with a mean (95% CI) bias of −3 (−18 to 11) ml/min for the combined groups. Daptomycin CLR significantly correlated (r2 = 0.3, P = 0.03) with daptomycin CLT. However, no significant relationship was noted between measured GFR, estimated GFR, or estimated CLCR and daptomycin CLR (liter/h).
Safety data.
No serious adverse events were reported during the study, and all subjects tolerated the daptomycin infusion. Two subjects complained of mild headache during the UNMHSC GCRC admission, and their symptoms resolved following administration of a single 650-mg dose of acetaminophen.
DISCUSSION
The pharmacokinetic profile of daptomycin has been characterized in healthy subjects across a dose range of 0.5 to 12 mg/kg (2, 10, 21, 25, 26). Population pharmacokinetic analyses have also quantitatively determined the influence of clinical variables such as age, sex, weight, renal function, and acute infection on the pharmacokinetics of daptomycin (9). These analyses have led to two fundamental principles when dosing daptomycin: (i) dosage interval adjustment based on renal function, as estimated by CLCR (Cockcroft-Gault), is necessary and (ii) daptomycin dosing based on TBW is the optimal approach.
The current study improves our knowledge of daptomycin dosing in morbid obesity through a more thorough analysis of renal function by measurement of GFR, estimation of GFR (four-variable MDRD), and estimation of CLCR using multiple body size descriptors (TBW, IBW, and FFW) in the Cockcroft-Gault equation. When considering the daptomycin dosing interval, the estimation of CLCR by the Cockcroft-Gault equation with TBW has been the recommended approach (CUBICIN package insert; Cubist Pharmaceuticals, Inc., Lexington, MA). As illustrated in Table 1, this method of evaluation inevitably leads to a gross overestimation of CLCR among morbidly obese subjects. Indeed, this overestimation was recognized in the daptomycin population pharmacokinetic analyses, where 38 subjects had CLCR levels estimated to be >150 ml/min, which were all set to 150 ml/min for modeling purposes (9). Consequently, the use of TBW should be considered inappropriate when calculating CLCR in morbidly obese subjects if the Cockcroft-Gault equation is used. Our study determined that the use of IBW in the Cockcroft-Gault equation or the use of the four-variable MDRD equation provided a more accurate reflection of GFR in this population. Ultimately, only the four-variable MDRD equation provided an unbiased estimate of GFR and demonstrated the best correlation with daptomycin CLT.
To date, one published study has provided comparative pharmacokinetic data for daptomycin in morbidly obese subjects. Dvorchik and Damphouse evaluated the pharmacokinetics of daptomycin dosed as 4 mg/kg of TBW in moderately obese, morbidly obese, and matched nonobese subjects (11). The subjects were matched by age (±10 years), sex, and renal function, defined as a CLCR of ≥70 ml/min using the Cockcroft-Gault equation and TBW. Given the inherent bias of this equation when using TBW, it is plausible that morbidly obese subjects with low GFRs could have been matched based on a presumption of equivalent renal function, e.g., a 40-year-old (120-kg) female subject with a serum creatinine of 2.0 mg/dl would have an estimated CLCR of ≥70 ml/min and could have been matched to a 40-year-old (60-kg) female subject with a serum creatinine of 1.0 mg/dl. Use of the four-variable MDRD equation in this scenario would confirm that the morbidly obese subject had a 50% lower GFR than the normal-weight control. Again, this intrinsic imprecision of equations to estimate GFR was addressed in the current study through a more accurate determination of GFR using [125I]sodium iothalamate CL and matching subjects solely on serum creatinine. As a result, our control subject matching criteria created groups that were closely matched for age, sex, race, and most importantly GFR.
We also determined that dosing daptomycin based on TBW resulted in higher (∼60% higher) plasma Cmax and AUC0-24 values in morbidly obese subjects than in normal-weight subjects. These higher values were explained by the higher total dose administered and were not secondary to differences in either V or CLT. Our results are in direct contrast to the data set previously published by Dvorchik and Damphouse (11). The mean absolute V and CLT values were approximately 50% higher in the morbidly obese group than in normal-weight controls in the previous study (11). In contrast, in the current study the mean absolute V was 22% higher and CLT 12% higher in the morbidly obese group than in the normal-weight group but the data did not reach a statistically significant level. One could argue that our improved control subject matching standards provided a better reflection of the lack of difference between these key pharmacokinetic variables (especially CLT) when comparing morbidly obese to normal-weight subjects.
Evidence of our improved control matching standards is further supported by a focused review of previous literature. A comparison of values for our morbidly obese group to those published by Dvorchik and Damphouse (11) reveals strikingly similar data. In contrast, when you compare the normal-weight control groups between their study and ours, a marked difference is notable. For example, the mean (±SD) Cmax in our normal-weight group was 42.3 ± 11.9 mg/liter, compared to 53.2 ± 6.0 mg/dl in the previous study, despite receipt of similar doses (4 mg/kg). Indeed, data from multiple studies suggest that a 4-mg/kg regimen leads to a mean Cmax concentration of 50 to 55 mg/liter in patients that weigh 60 to 80 kg (10, 25, 26). However, a key difference between the current study and data published by Dvorchik and Damphouse (11) is that we included only females in our subject population. The subject population studied by Dvorchik and Damphouse (11) was divided as follows: moderately obese (six females), morbidly obese (four males and three females), and matched nonobese controls (four males and eight females). Consequently, when Dvorchik and Damphouse (11) compared their moderately obese group to the matched nonobese group they compared female subjects only. In this comparison, the mean Cmax decreased from 53.2 mg/liter (males and females) to 46.3 mg/liter (females only), implying that female subjects had lower Cmax values secondary to receipt of lower total doses, presumably because of lower TBW (11). Similarly, another study published by Dvorchik and Damphouse, which evaluated daptomycin (4 mg/kg of TBW) pharmacokinetics in a young (23.8 ± 4.3 years) predominantly female group, revealed a mean (±SD) Cmax of 42.3 ± 6.5 mg/liter (8). As a result, our data imply that dosing daptomycin based on TBW will lead to higher Cmax and AUC0-24 values in morbidly obese subjects than in normal-weight subjects with similar GFR values.
The implications of higher Cmax and AUC values may not necessarily be harmful for an antimicrobial such as daptomycin, which displays a concentration-dependent pharmacodynamic profile (7, 17, 22), i.e., dosing daptomycin on an alternate body size descriptor such as FFW or IBW may yield lower Cmax values. Given that the expected Cmax is dependent on V, consideration of the relationship of V to weight is important. We found relationships of V to weight similar to those published by Dvorchik and Damphouse (11). There was a significant and good correlation (P < 0.05) between absolute V, BMI (r2 = 0.52), and TBW (r2 = 0.66). We also found a relatively poor correlation between absolute V and IBW (r2 = 0.0004) and better correlation of V with FFW (r2 = 0.32). This evaluation is especially important given that the absolute V of daptomycin has been documented to increase by 37% during acute bacterial infections, implying even lower Cmax values with the standard 4-mg/kg dosing approach if FFW or IBW is used as the dosing weight (9). Therefore, we would argue that the approach of dosing daptomycin based on TBW should still be considered appropriate for morbidly obese subjects.
From a safety perspective, the relevance of the dosing interval may be more important given that high C24 values may be associated with an increased risk of CPK elevation. Bhavnani and colleagues used data from 120 patients with bacteremia and/or endocarditis treated with daptomycin to determine the predictive values of AUC0-24, Cmax, and C24 on CPK elevation (3). Based on this analysis, patients treated with daptomycin with a resultant C24 of ≥25.7 mg/liter would have a 14% probability of CPK elevation after 7 days of therapy. Given the strong association between GFR and daptomycin CLT, estimation of GFR is an important consideration when choosing the daptomycin dosing interval which will impact C24 values. We have demonstrated that estimation of GFR through the surrogate CLCR using the Cockcroft-Gault equation and TBW is inappropriate for morbidly obese subjects and that IBW is a better approach in this population. Although the current study did not specifically include populations of morbidly obese subjects with reduced renal function, we have previously published a case illustrating this point (20). Thus, caution is warranted when dosing daptomycin in morbidly obese subjects with chronic renal insufficiency.
The current study did not evaluate the impact of renal insufficiency on the pharmacokinetic profile of daptomycin in morbidly obese subjects. Also, our population included healthy subjects, which are not entirely reflective of patients treated with daptomycin. Future study of these populations will be essential given that obesity may predispose patients to develop renal insufficiency (4). Our study population was well matched, as previously noted; however, it ultimately included only female subjects. Despite this limitation, the premise of not using TBW to estimate CLCR by the Cockcroft-Gault equation is probably true for male morbidly obese subjects. Finally, we assessed plasma concentrations and not tissue or interstitial concentrations, which are more important when considering management of skin and skin structure infections. Despite these limitations, the current study incorporated a systematic approach to define the influence of obesity on the pharmacokinetics of daptomycin and assessed the value of the four-variable MDRD equation to define renal function in morbidly obese subjects.
In summary, the current study evaluated the single-dose pharmacokinetic profile of daptomycin in morbidly obese subjects and a well-matched (by age, sex, race, serum creatinine, and GFR) normal-weight control group. Dosing of daptomycin based on TBW resulted in higher Cmax and AUC values in the morbidly obese group than in the normal-weight group, and both sets of values were associated with higher total doses received in the morbidly obese group. The absolute V and CLT values were not significantly different between the two groups. However, the correlation between TBW and V was good whereas IBW and V had a very poor correlation, implying that weight-based dosing on TBW is appropriate. In contrast, use of TBW to determined CLCR by the Cockcroft-Gault equation overestimated GFR in morbidly obese subjects. Use of the four-variable MDRD equation or the Cockcroft-Gault equation using IBW provided an unbiased estimate of GFR. The four-variable MDRD equation also demonstrated the best correlation with daptomycin CLT. Further pharmacokinetic studies of morbidly obese patients with acute bacterial infections or chronic renal insufficiency, as well as quantification of interstitial concentrations, will be necessary to optimize the safety and efficacy of daptomycin in this emerging population.
Acknowledgments
The study was supported by an independent research grant agreement with Cubist Pharmaceuticals, Inc. A portion of this work was supported by the University of New Mexico Clinical Research Center, which is supported by the National Institutes of Health (M01 RR-00997).
Footnotes
Published ahead of print on 4 June 2007.
REFERENCES
- 1.Bamgbade, O. A., T. W. Rutter, O. O. Nafiu, and P. Dorje. 2006. Postoperative complications in obese and nonobese patients. World J. Surg. 31:556-560. [DOI] [PubMed] [Google Scholar]
- 2.Benvenuto, M., D. P. Benziger, S. Yankelev, and G. Vigliani. 2006. Pharmacokinetics and tolerability of daptomycin at doses up to 12 milligrams per kilogram of body weight once daily in healthy subjects. Antimicrob. Agents Chemother. 50:3245-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhavnani, S. M., P. G. Ambrose, F. B. Oleson, and G. L. Drusano. 2006. Toxicodynamics of daptomycin in patients with bacteremia and/or endocarditis. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-655.
- 4.Bosma, R. J., J. A. Krikken, J. J. Homan van der Heide, P. E. de Jong, and G. J. Navis. 2006. Obesity and renal hemodynamics. Contrib. Nephrol. 151:184-202. [DOI] [PubMed] [Google Scholar]
- 5.Cockcroft, D. W., and M. H. Gault. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31-41. [DOI] [PubMed] [Google Scholar]
- 6.Cohen, M. L., F. G. Smith, Jr., R. S. Mindell, and R. L. Vernier. 1969. A simple, reliable method of measuring glomerular filtration rate using single, low dose sodium iothalamate I-131. Pediatrics 43:407-415. [PubMed] [Google Scholar]
- 7.Dandekar, P. K., P. R. Tessier, P. Williams, C. H. Nightingale, and D. P. Nicolau. 2003. Pharmacodynamic profile of daptomycin against Enterococcus species and methicillin-resistant Staphylococcus aureus in a murine thigh infection model. J. Antimicrob. Chemother. 52:405-411. [DOI] [PubMed] [Google Scholar]
- 8.Dvorchik, B., and D. Damphouse. 2004. Single-dose pharmacokinetics of daptomycin in young and geriatric subjects. J. Clin. Pharmacol. 44:612-620. [DOI] [PubMed] [Google Scholar]
- 9.Dvorchik, B., R. D. Arbeit, J. Chung, S. Liu, W. Knebel, and H. Kastrissios. 2004. Population pharmacokinetics of daptomycin. Antimicrob. Agents Chemother. 48:2799-2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dvorchik, B. H., D. Brazier, M. F. DeBruin, and R. D. Arbeit. 2003. Daptomycin pharmacokinetics and safety following administration of escalating doses once daily to healthy subjects. Antimicrob. Agents Chemother. 47:1318-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dvorchik, B. H., and D. Damphouse. 2005. The pharmacokinetics of daptomycin in moderately obese, morbidly obese, and matched nonobese subjects. J. Clin. Pharmacol. 45:48-56. [DOI] [PubMed] [Google Scholar]
- 12.Edmiston, C. E., C. Krepel, H. Kelly, J. Larson, D. Andris, C. Hennen, A. Nakeeb, and J. R. Wallace. 2004. Perioperative antibiotic prophylaxis in the gastric bypass patient: do we achieve therapeutic levels? Surgery 136:738-747. [DOI] [PubMed] [Google Scholar]
- 13.Flegal, K. M., M. D. Carroll, C. L. Ogden, and C. L. Johnson. 2002. Prevalence and trends in obesity among US adults, 1999-2000. JAMA 288:1723-1727. [DOI] [PubMed] [Google Scholar]
- 14.Heath, E. M., T. D. Adams, M. M. Daines, and S. C. Hunt. 1998. Bioelectric impedance and hydrostatic weighing with and without head submersion in persons who are morbidly obese. J. Am. Diet. Assoc. 98:869-875. [DOI] [PubMed] [Google Scholar]
- 15.Hollenstein, U. M., M. Brunner, R. Schmid, and M. Muller. 2001. Soft tissue concentrations of ciprofloxacin in obese and lean subjects following weight-adjusted dosing. Int. J. Obes. Relat. Metab. Disord. 25:354-358. [DOI] [PubMed] [Google Scholar]
- 16.Levey, A. S., J. Coresh, T. Greene, L. A. Stevens, Y. L. Zhang, S. Hendriksen, J. W. Kusek, F. Van Lente, et al. 2006. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann. Intern. Med. 145:247-254. [DOI] [PubMed] [Google Scholar]
- 17.Louie, A., P. Kaw, W. Liu, N. Jumbe, M. H. Miller, and G. L. Drusano. 2001. Pharmacodynamics of daptomycin in a murine thigh model of Staphylococcus aureus infection. Antimicrob. Agents Chemother. 45:845-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myles, T. D., J. Gooch, and J. Santolaya. 2002. Obesity as an independent risk factor for infectious morbidity in patients who undergo cesarean delivery. Obstet. Gynecol. 100:959-964. [DOI] [PubMed] [Google Scholar]
- 19.Nasraway, S. A., Jr., M. Albert, A. M. Donnelly, R. Ruthazer, S. A. Shikora, and E. Saltzman. 2006. Morbid obesity is an independent determinant of death among surgical critically ill patients. Crit. Care Med. 34:964-970. [DOI] [PubMed] [Google Scholar]
- 20.Pai, M. P., R. C. Mercier, and S. E. Allen. 2006. Using vancomycin concentrations for dosing daptomycin in a morbidly obese patient with renal insufficiency. Ann. Pharmacother. 40:553-558. [DOI] [PubMed] [Google Scholar]
- 21.Rybak, M. J., E. M. Bailey, K. C. Lamp, and G. W. Kaatz. 1992. Pharmacokinetics and bactericidal rates of daptomycin and vancomycin in intravenous drug abusers being treated for gram-positive endocarditis and bacteremia. Antimicrob. Agents Chemother. 36:1109-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Safdar, N., D. Andes, and W. A. Craig. 2004. In vivo pharmacodynamic activity of daptomycin. Antimicrob. Agents Chemother. 48:63-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spinler, S. A., J. J. Nawarskas, E. G. Boyce, J. E. Connors, S. L. Charland, S. Goldfarb, et al. 1998. Predictive performance of ten equations for estimating creatinine clearance in cardiac patients. Ann. Pharmacother. 32:1275-1283. [DOI] [PubMed] [Google Scholar]
- 24.Tett, S. E., C. M. Kirkpatrick, A. S. Gross, and A. J. McLachlan. 2003. Principles and clinical application of assessing alterations in renal elimination pathways. Clin. Pharmacokinet. 42:1193-1211. [DOI] [PubMed] [Google Scholar]
- 25.Wise, R., T. Gee, J. M. Andrews, B. Dvorchik, and G. Marshall. 2002. Pharmacokinetics and inflammatory fluid penetration of intravenous daptomycin in subjects. Antimicrob. Agents Chemother. 46:31-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woodworth, J. R., E. H. Nyhart, Jr., G. L. Brier, J. D. Wolny, and H. R. Black. 1992. Single-dose pharmacokinetics and antibacterial activity of daptomycin, a new lipopeptide antibiotic, in healthy subjects. Antimicrob. Agents Chemother. 36:318-325. [DOI] [PMC free article] [PubMed] [Google Scholar]