Abstract
UV light inactivation of Mycobacterium avium subsp. paratuberculosis in Middlebrook 7H9 broth and whole and semiskim milk was investigated using a laboratory-scale UV machine that incorporated static mixers within UV-penetrable pipes. UV treatment proved to be less effective in killing M. avium subsp. paratuberculosis suspended in milk (0.5- to 1.0-log10 reduction per 1,000 mJ/ml) than that suspended in Middlebrook 7H9 broth (2.5- to 3.3-log10 reduction per 1,000 mJ/ml). The FASTPlaqueTB phage assay provided more rapid enumeration of surviving M. avium subsp. paratuberculosis (within 24 h) than culture on Herrold's egg yolk medium (6 to 8 weeks). Despite the fact that plaque counts were consistently 1 to 2 log10 lower than colony counts throughout the study, UV inactivation rates for M. avium subsp. paratuberculosis derived using the phage assay and culture results were not significantly different (P = 0.077).
UV light has been used for the disinfection of drinking water and wastewater systems to inactivate potential waterborne pathogens for many years (2). UV light at wavelengths shorter than 280 nm (termed UV-C) has a germicidal effect on most types of microorganisms by formation of thymine dimers in DNA and RNA that inhibit transcription and replication of nucleic acids, thereby rendering the microorganism unable to reproduce (12, 17). UV technology is an emerging nonthermal process for food preservation (12), although there has been limited research to date on the use of UV for the inactivation of bacteria and viruses in liquid foods, such as fruit juices and milk. UV treatment has been shown to result in significant reductions in numbers of Escherichia coli, E. coli O157:H7, and Cryptosporidium parvum bacteria in apple cider (14, 20, 27). In relation to UV treatment of milk, Matak et al. (18) reported a >5-log10 reduction in Listeria monocytogenes numbers in goat's milk by exposure to a cumulative UV dose of 15.8 ± 1.6 mJ/cm2, and Reinemann et al. (21) reported that UV treatment of 15 kJ/liter achieved a 3-log10 reduction in total numbers of bacteria present in raw cow's milk, with coliforms showing the greatest reduction in numbers and spore formers showing only a modest reduction.
Mycobacterium avium subsp. paratuberculosis is a gram-positive, acid-fast bacterium characterized by its extremely low growth rate and its requirement for the presence of mycobactin J, an iron-chelating compound, in culture media used to recover it (11). M. avium subsp. paratuberculosis is the causative agent of Johne's disease in ruminant animals, most commonly cattle (15). Due to the similarities between the symptoms and pathological changes in the guts of humans with Crohn's disease and those that occur in cattle with Johne's disease, some involvement for M. avium subsp. paratuberculosis in Crohn's disease has been proposed (5, 16), although the association between M. avium subsp. paratuberculosis and Crohn's disease remains a controversial subject (3, 7).
If M. avium subsp. paratuberculosis is implicated in Crohn's disease, a possible route of transmission of M. avium subsp. paratuberculosis to humans is via cows' milk (5, 7). Therefore, it is essential to have an effective method for the elimination of M. avium subsp. paratuberculosis from milk. Most milk is high temperature, short time (HTST) pasteurized (71.7°C/15 s) before human consumption. Previous studies have shown that small numbers of M. avium subsp. paratuberculosis cells may survive HTST pasteurization, especially if the bacterium is initially present in high numbers (8, 9, 13, 23, 24). As HTST pasteurization may not achieve the complete elimination of viable M. avium subsp. paratuberculosis from milk, alternative methods of milk processing are being sought by the dairy industry. UV treatment may represent such an alternative process.
The amount of microbial damage caused, and hence the log10 reduction achieved, by UV light depends on the resistance of the organism to UV light, the absorptive properties of the medium in which the organism is suspended, and the UV dose applied. There is a distinct possibility that milk solids may limit UV penetration and thereby decrease the efficacy of UV treatment (12). In order to maximize UV penetration, Iatros Limited, Dundee, Scotland, has designed and manufactured a laboratory-scale UV machine incorporating a series of static mixers within UV-C-penetrable pipes. The static mixer consists of a series of alternating right- and left-hand helical elements with 180° rotations, each juxtaposed at 90° to the element preceding it. The static mixers achieve flow division, flow reversal, radial mixing, and axial differentiation of the fluid stream, which constantly changes the thin film at the inner wall of the pipe, thereby exposing more bacterial cells to the UV light during treatment. This study was undertaken to investigate the inactivation of M. avium subsp. paratuberculosis by UV light using the Iatros laboratory-scale UV machine. Initial experiments were carried out with M. avium subsp. paratuberculosis in Middlebrook 7H9 broth to establish the UV inactivation kinetics of three different M. avium subsp. paratuberculosis strains. Subsequent experiments were conducted using ultra-heat-treated (UHT) semiskim and whole milk inoculated with M. avium subsp. paratuberculosis to assess the impact of milk components on the efficacy of UV treatment. Surviving M. avium subsp. paratuberculosis bacteria were enumerated by conventional culture with Herrold's egg yolk medium and by a commercially available phage amplification assay, FASTPlaqueTB (Biotec Laboratories Limited, Ipswich, United Kingdom), which has recently been evaluated by Stanley and coworkers at the University of Nottingham for detection of M. avium subsp. paratuberculosis in milk (25, 26; E. Stanley, R. Mole, and C. Rees, poster presentation at the 104th Annual General Meeting of the American Society for Microbiology, New Orleans, LA, May 2004). Fuller details of the phage assay are provided below.
M. avium subsp. paratuberculosis strains and preparation of inoculum.
Three strains of M. avium subsp. paratuberculosis were included in this study: NCTC 8578, 806R, and 796PSS. NCTC 8578 is a bovine type strain originally isolated from feces. 806R and 796PSS were previously isolated in our laboratory from raw and commercially pasteurized cows' milk, respectively. M. avium subsp. paratuberculosis strains were grown in 200 ml Middlebrook 7H9 broth containing 10% oleic acid-albumin-dextrose-catalase (both from Difco), 2 μg/ml mycobactin J (Synbiotics Europe SAS, Lyon, France), and 0.002% glycerol (Sigma). Glycerol was added to the 7H9 broth rather than Tween 80 because the latter is known to interfere with phage attachment (25). The cultures were incubated for 6 weeks at 37°C prior to use in UV experiments.
Operation of the laboratory-scale UV machine.
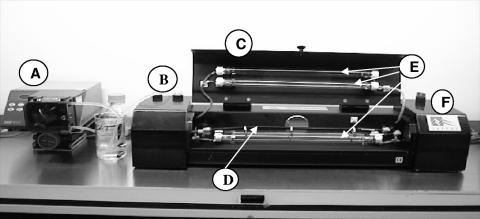
The Iatros laboratory-scale UV machine consists of four UV lamps surrounding a UV-penetrable flow tube incorporating static mixers housed within an irradiation chamber plus an associated cooling fan (Fig. 1). A flow rate of 168 ml/min through the flow tube was achieved by an external Watson-Marlow peristaltic pump set at 100 rpm. For health and safety reasons, both the UV machine and the pump were housed inside a class I safety cabinet within a containment level 2 pathogen laboratory for the duration of this study involving M. avium subsp. paratuberculosis.
FIG. 1.
UV machine (Iatros Limited, Dundee, Scotland) and associated pump inside class 1 safety cabinet (A, peristaltic pump; B, UV lamp off/on switches; C, irradiation chamber lid; D, UV-penetrable tube incorporating static mixers; E, four UV lamps; F, master off/on switch and fan housing).
Before each experiment, the UV lamp surfaces were cleaned with isopropyl alcohol and lint-free tissue. Prior to each use, the machine was flushed with three changes of tap water (5- to 10-min cycles) and a final cycle of sterile distilled water for 30 min, with the UV lamps switched on. The tubes were emptied of distilled water before each experiment commenced, and the first 60 ml (three times the internal volume of the tube) of liquid emanating from the system was discarded to ensure no dilution of broth culture or spiked milk with residual water. After completion of each UV inactivation experiment, gross broth or milk residues were flushed from the tubes with tap water by briefly increasing the flow rate to the maximum setting (220 rpm). NaOH (0.5 M) was then circulated through the machine, with the UV lamps on, for 2 h before the machine and pump were switched off. The NaOH solution was left in the system until the machine was next required.
Estimation of cumulative UV dose rates.
The absorbance at 352 nm of the 1% sodium iodide (NaI) actinometer solution collected after each pass through the UV machine was measured 60 min following UV exposure, using a Jenway 6305 spectrophotometer (Barloworld Scientific Limited, Dunmow, Essex, England). The cumulative UV dose received by the sample was derived from NaI actinometry calibration curves provided by Iatros Limited, Dundee, Scotland.
Inactivation of M. avium subsp. paratuberculosis in Middlebrook 7H9 broth.
Each 200-ml, 6-week-old 7H9 broth culture of M. avium subsp. paratuberculosis was diluted to 400 ml (working volume) by the addition of fresh Middlebrook 7H9 broth containing 10% oleic acid-albumin-dextrose-catalase supplement and mixed thoroughly by shaking it before use. The entire 400 ml broth culture was passed through the UV machine, with the lamps switched on, at a fixed flow rate of approximately 168 ml/min and collected into a sterile empty glass bottle (one pass). This process was then repeated up to 11 more times, with the UV-treated broth culture after each pass acting as the inoculum for the subsequent pass. Samples (ca. 10 ml) for testing were collected from the outlet tube after 1, 2, 4, 8, and 12 passes through the machine, corresponding to UV dose rates of 200, 320, 530, 960, and 1,320 mJ/ml, respectively. Results for the first experiments with Middlebrook 7H9 broth cultures suggested that the natural tendency of M. avium subsp. paratuberculosis to exist as sizeable clumps in broth cultures was affecting UV inactivation kinetics, resulting in a rapid initial decrease in numbers of viable M. avium subsp. paratuberculosis bacteria followed by a tailing of the inactivation curve when little further reduction in numbers occurred. The potential capability of the static mixers in the UV machine to declump M. avium subsp. paratuberculosis suspensions was investigated by passing 400 ml of M. avium subsp. paratuberculosis 796PSS broth culture through the UV machine for 1, 2, 3, 4, and 5 min, with the UV lamps switched off. Results indicated a maximal increase (0.7 log10) in numbers of M. avium subsp. paratuberculosis bacteria after 2 min of circulation through the static mixers which was equivalent to the increase in numbers observed upon the declumping of a 10-ml portion of the same culture by vortexing it with five 3-mm glass beads for 2 min. In our experience, a 0.5- to 1.0-log10 increase in M. avium subsp. paratuberculosis numbers is typically achieved by declumping. Further UV inactivation experiments involving Middlebrook 7H9 broth cultures and subsequent experiments involving M. avium subsp. paratuberculosis-inoculated milk were therefore conducted after M. avium subsp. paratuberculosis cells were declumped for 2 min by circulation through the UV machine with the lamps off. Two independent runs were carried out for each M. avium subsp. paratuberculosis strain by using the “multiple-pass” method. Appropriate dilutions of each sample were prepared in Middlebrook 7H9 broth, and the numbers of viable M. avium subsp. paratuberculosis bacteria surviving UV treatment were enumerated as described below.
UV inactivation of M. avium subsp. paratuberculosis in milk.
UHT whole and semiskim cows' milk was purchased from a local supermarket. UV inactivation experiments using UHT whole or semiskim milk were conducted as for Middlebrook 7H9 broth with the following alterations. Each 200-ml, 6-week-old broth culture of M. avium subsp. paratuberculosis was centrifuged in four 50-ml aliquots at 2,500 × g for 15 min. The resulting pellets were resuspended in 5 ml Middlebrook 7H9 broth and then added to 400 ml UHT whole or semiskim milk. After vigorous shaking to disperse the inoculum, M. avium subsp. paratuberculosis cells in the inoculated milk were declumped by circulating it through the UV machine, with the lamps switched off, for 2 min. A 5-ml control (pass 0) sample was collected for testing after the declumping. The inoculated milk was then passed through the UV machine for 16 successive passes, and 10-ml samples were collected after 2, 4, 8, 12, and 16 passes through the machine, corresponding to UV dose rates of 530, 960, 1,700, 2,270, and 2,860 mJ/ml, respectively. Two independent runs were carried out for each M. avium subsp. paratuberculosis strain in both types of milk. As milk components are known to interfere with the phage in the FASTPlaqueTB assay (25), 5 ml of each untreated or UV-treated milk sample was centrifuged at 2,500 × g for 15 min and the pellet resuspended in 5 ml Middlebrook 7H9 broth before the appropriate dilutions were prepared, also in Middlebrook 7H9 broth. Enumeration of M. avium subsp. paratuberculosis cells was carried out as described below.
Enumeration of viable M. avium subsp. paratuberculosis cells before and after UV treatment.
M. avium subsp. paratuberculosis bacteria in broth and milk were enumerated before and immediately after UV treatment by two methods.
(i) FASTPlaqueTB assay.
The FASTPlaqueTB phage amplification assay (Biotec Laboratories Limited, Ipswich, United Kingdom), which was originally developed as a rapid bacteriophage assay for determination of the Mycobacterium tuberculosis complex in decontaminated sputum samples, was employed to obtain a viable M. avium subsp. paratuberculosis count within 24 h (25; E. Stanley, R. Mole, and C. Rees, poster presentation at the 104th Annual General Meeting of the American Society for Microbiology, New Orleans, LA, May 2004). The FASTPlaqueTB assay, which consists of sufficient lyophilized Actiphage and sensor cells, Virusol (viricide), broth, growth supplement, agar, and vials for 50 tests, was used according to the manufacturer's instructions. Duplicate 1-ml aliquots of three appropriate dilutions of each Middlebrook 7H9 broth culture or inoculated milk or UV-treated sample were incubated with Actiphage at 37°C for 1 h, treated with Virusol for 5 min, and mixed with rapidly growing “sensor” cells (Mycobacterium smegmatis), and the entire sample was transferred to an empty petri dish, to which 5 ml molten (55°C) FASTPlaque agar was added. Afterward, the solidified plates were incubated at 37°C overnight (18 h) before the number of plaques present (equivalent to the number of viable M. avium subsp. paratuberculosis bacteria present in the sample) was counted and expressed as the number of PFU per ml. This method provided a rapid viable count of M. avium subsp. paratuberculosis cells surviving UV treatment. The fact that results were available so quickly meant that if the dilutions chosen for testing during the first experiments with 7H9 broth and milk were not appropriate and counts were missed, the dilutions chosen in subsequent experiments could be modified. In cases where dilutions were missed and a full set of data was not obtained, the experiment was repeated.
(ii) Colony counts.
Duplicate 0.1-ml aliquots of appropriate dilutions of the Middlebrook 7H9 broth culture or inoculated milk or UV-treated samples were inoculated onto Herrold's egg yolk medium containing 2 μg/ml mycobactin J (HEYM) dispensed in 50-mm petri dishes by using the spread plate technique. HEYM plates were wrapped in Duraseal laboratory sealing film (Diversified Biotech, Boston) to prevent them from drying out during the lengthy incubation period. The plates were incubated at 37°C for 6 to 8 weeks before a colony count (CFU/ml) was obtained.
Plaque (PFU/ml) and colony (CFU/ml) counts, and log10 reduction data derived using these counts, were compared by analysis of variance and regression analysis (against UV level) using GenStat release 8.2.
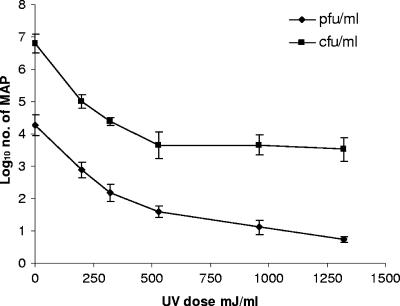
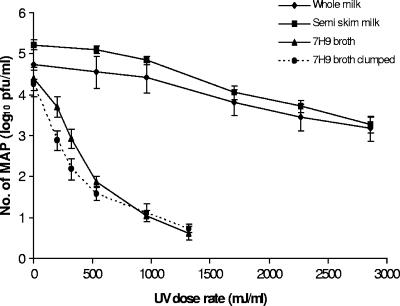
Initial UV inactivation experiments with clumped Middlebrook 7H9 broth cultures of the three M. avium subsp. paratuberculosis strains resulted in nonlinear UV inactivation curves (mean r2 value = 0.64; range, 0.42 to 0.82) showing a rapid decline in numbers at the beginning of the treatment, with tailing at higher doses (Fig. 2 and 3). The shapes of the UV inactivation curves obtained for clumped M. avium subsp. paratuberculosis cells were very reminiscent of those previously obtained for heat-treated M. avium subsp. paratuberculosis (9). Tailing of thermal inactivation curves of M. avium subsp. paratuberculosis has been attributed to the existence of cells in clumps (10), so a similar explanation was postulated for the shapes of UV inactivation curves for clumped broth cultures. Attempts were therefore made to find a method for declumping the 400-ml Middlebrook 7H9 broth suspensions before UV treatment. Most of the published declumping protocols for mycobacteria (such as vortexing with glass beads, sonication, and homogenization) are applicable only to small volumes of broth culture and were not appropriate for declumping a 400-ml volume of broth culture required for the UV experiments. Trials demonstrated that the static mixers in the flow tube of the UV machine could break up clumps of M. avium subsp. paratuberculosis cells by circulating suspensions (broth or milk) through the UV machine for 2 min, with the lamps switched off. When Middlebrook 7H9 broth cultures of M. avium subsp. paratuberculosis declumped in this way were subsequently UV treated, inactivation curves showed a more linear relationship (mean r2 value = 0.9; range, 0.88 to 0.92) between the number of surviving M. avium subsp. paratuberculosis bacteria and the UV dose rate (Fig. 3). A 3- to 4-log10 reduction in declumped M. avium subsp. paratuberculosis cells in Middlebrook 7H9 broth was achieved after 12 passes through pipe 25, which represents a maximum cumulative UV dose rate of approximately 1,320 mJ/ml.
FIG. 2.
UV inactivation of clumped M. avium subsp. paratuberculosis bacteria (MAP) in Middlebrook 7H9 broth as assessed by a FASTPlaqueTB assay (PFU/ml) and by culture on HEYM (CFU/ml).
FIG. 3.
Impact of UV dose rate on inactivation of clumped M. avium subsp. paratuberculosis bacteria (MAP) in Middlebrook 7H9 broth and declumped M. avium subsp. paratuberculosis bacteria in Middlebrook 7H9 broth, whole milk, and semiskim milk. Combined plaque count data for three M. avium subsp. paratuberculosis strains are presented, and error bars consistently represent standard errors of the means of six counts.
The whole milk used in these experiments contained 4% fat and the semiskim milk, 1.7% fat. UHT milk was employed so that there would be no interference in colony or phage counts from background microflora in the milk. Linear UV inactivation curves were generally obtained for M. avium subsp. paratuberculosis in milk (Fig. 3). There was no significant difference in the responses of the three M. avium subsp. paratuberculosis strains to UV treatment (P = 0.45). However, the three M. avium subsp. paratuberculosis strains were found to be much more resistant to UV inactivation when suspended in milk, whole or semiskim, than in Middlebrook 7H9 broth (P < 0.001) (Fig. 3). The highest UV dose rate applied (approximately 2,860 mJ/ml) achieved similar mean reductions in M. avium subsp. paratuberculosis in whole and semiskim milk of 1.5 to 2.6 log10 and 1.7 to 2.8 log10, respectively (P = 0.258). As the fat content of the milk was not observed to alter UV sensitivity, UV penetration into milk, and hence M. avium subsp. paratuberculosis inactivation achieved in milk compared to that achieved in 7H9 broth, must have been reduced by the presence of milk proteins. This was not unexpected, since milk is opaque and has a high absorption coefficient at 254 nm (which is used to measure the protein content of solutions) of 300 cm−1 (22), whereas Middlebrook 7H9 broth is a translucent, straw-colored liquid.
There have been only two studies to date on the impact of UV treatment on microorganisms in milk (18, 21). Matak et al. (18) reported that a cumulative UV dose of 15.8 ± 1.6 mJ/cm2 achieved a >5-log10 reduction in Listeria monocytogenes in goat's milk with the use of a CiderSure 3500 UV apparatus, manufactured by FPE Inc., Macedon, NY. The different dose rate units used (mJ/cm2 rather than mJ/ml) make it difficult for us to comment on the relative resistance levels of M. avium subsp. paratuberculosis and L. monocytogenes. In contrast, Reinemann et al. (21), using the PureUV system (PureUV, South Africa), showed that a UV dose rate of 1 kJ/liter (equivalent to 1,000 mJ/ml) resulted in mean log10 reductions of 1.92, 2.08, 1.42, 2.48, 2.53, and 0.36 in standard plate counts, psychrotrophs, thermophiles, coliforms, E. coli bacteria, and spore-forming bacteria, respectively, in raw cows' milk. As the dose rate units used by Reinemann et al. (21) were the same as those used in this study, it is possible to directly compare their findings with our results. The log10 kill rates for M. avium subsp. paratuberculosis achieved per 1,000-mJ/ml UV dose in Middlebrook 7H9 broth and semiskim and whole milk are presented in Table 1. In Middlebrook 7H9 broth, a kill rate of 2.5 to 3.3 log10 per 1,000-mJ/ml UV dose was achieved, depending on the method used to enumerate survivors and the M. avium subsp. paratuberculosis strain studied, whereas the kill rate achieved for semiskim or whole milk was 0.5 to 1.0 log10 per 1,000-mJ/ml UV dose. These data indicate that M. avium subsp. paratuberculosis is much more UV resistant than all the types of milk microorganisms studied by Reinemann et al. (21) previously, with the exception of spore formers. This is not surprising, given that other Mycobacterium spp. have previously been shown to be considerably more UV resistant than other bacterial genera when treated in drinking water and air (4, 12, 19). Potential photoreactivation of M. avium subsp. paratuberculosis after UV treatment, which has previously been reported for other Mycobacterium spp. (1, 6), was not investigated during this study. If photoreactivation were to occur in M. avium subsp. paratuberculosis, then the net log10 inactivation achievable by UV treatment would be even less than that observed here.
TABLE 1.
UV inactivation rates for declumped M. avium subsp. paratuberculosis cells suspended in different substrates as determined by the FASTPlaqueTB phage assay and culture on HEYMa
| M. avium subsp. paratuberculosis strain | Enumeration method | Mean log10 reduction per 1,000-mJ/ml UV dose for indicated test substrate
|
||
|---|---|---|---|---|
| Middlebrook 7H9 broth | Semiskim milk | Whole milk | ||
| 806R | Phage assay | 2.56 | 0.59 | 0.62 |
| Culture | 2.70 | 0.82 | 0.93 | |
| 796PSS | Phage assay | 2.64 | 0.72 | 0.52 |
| Culture | 3.07 | 0.74 | 0.91 | |
| NCTC 8578 | Phage assay | 3.26 | 0.75 | 0.52 |
| Culture | 2.92 | 1.01 | 0.87 | |
Results are means for two independent runs.
Significant differences between the colony counts (CFU/ml) and plaque counts (PFU/ml) were observed throughout this study (P < 0.001), the colony counts being consistently 1 to 2 log10 higher than the plaque counts (Fig. 2). This difference between PFU and CFU counts has been observed previously with M. tuberculosis by the manufacturer of the FASTPlaqueTB assay. Possible explanations for the lower plaque counts suggested by personnel at Biotec Laboratories Limited include an intrinsic property of the M. avium subsp. paratuberculosis-phage relationship, a characteristic of the assay which was originally optimized for M. smegmatis, and a cell state issue (André Trollip and Richard Mole, personal communication). However, the overall trends in UV inactivation of M. avium subsp. paratuberculosis indicated by the two methods of enumeration were not significantly different (P = 0.077), as indicated by the parallel inactivation curves in Fig. 2. The distinct advantage of the FASTPlaqueTB phage amplification assay is that a much more rapid (<24 h) indication of UV inactivation rates for M. avium subsp. paratuberculosis in broth and milk is obtained than by conventional culture (6 to 8 weeks). However, the current lack of correlation between PFU and CFU counts is a disadvantage that would affect detection sensitivity in a situation where low numbers of M. avium subsp. paratuberculosis bacteria were present.
In conclusion, the objective of this study was to determine the potential ability of UV treatment to inactivate M. avium subsp. paratuberculosis in milk. Results indicate that M. avium subsp. paratuberculosis is more resistant to UV light than other milk microorganisms studied to date and that only a 0.5- to 1-log10 reduction in M. avium subsp. paratuberculosis in whole or semiskim milk would be achieved by a dose of 1,000 mJ/ml, which is thought to be the dose limit before unacceptable organoleptic changes occur in milk. Consequently, UV treatment of milk would appear to have a limited ability to reduce numbers of M. avium subsp. paratuberculosis bacteria and is therefore not a viable alternative to current pasteurization processes for liquid milk, which achieve approximately 4-log10 reductions in M. avium subsp. paratuberculosis.
Acknowledgments
Leslie Altic was the recipient of a “Students into Work” grant from the Society for Applied Microbiology.
We thank Andrew Gunn, David Mowat, and Ian Cameron of Iatros Limited, Dundee, Scotland, for providing the laboratory-scale UV machine and training for its use; Duncan Pepper for assistance in determining UV dose rates; and David Kilpatrick for carrying out the statistical analyses.
Footnotes
Published ahead of print on 13 April 2007.
REFERENCES
- 1.Bohrerova, Z., and K. G. Linden. 2006. Assessment of DNA damage and repair in Mycobacterium terrae after exposure to UV irradiation. J. Appl. Microbiol. 101:995-1001. [DOI] [PubMed] [Google Scholar]
- 2.Bolton, J. R. 2001. Ultraviolet applications handbook, 2nd ed. Bolton Photosciences Inc., Edmonton, Canada.
- 3.Chacon, O., L. E. Bermudez, and R. G. Barletta. 2004. Johne's disease, inflammatory bowel disease and Mycobacterium paratuberculosis. Annu. Rev. Microbiol. 58:329-363. [DOI] [PubMed] [Google Scholar]
- 4.Collins, F. M. 1971. Relative susceptibility of acid-fast and non-acid-fast bacteria to ultraviolet light. Appl. Microbiol. 21:411-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins, M. T. 1997. Mycobacterium paratuberculosis: a potential foodborne pathogen? J. Dairy Sci. 80:3445-3448. [DOI] [PubMed] [Google Scholar]
- 6.David, H. L., W. D. Jones, and C. M. Newman. 1971. Ultraviolet light inactivation and photoreactivation in the mycobacteria. Infect. Immun. 4:318-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant, I. R. 2005. Zoonotic potential of Mycobacterium avium subsp. paratuberculosis: the current position. J. Appl. Microbiol. 98:1282-1293. [DOI] [PubMed] [Google Scholar]
- 8.Grant, I. R., E. I. Hitchings, A. McCartney, F. Ferguson, and M. T. Rowe. 2002. Effect of commercial-scale HTST pasteurization on the viability of Mycobacterium paratuberculosis in naturally infected cows' milk. Appl. Environ. Microbiol. 68:602-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grant, I. R., H. J. Ball, S. D. Neill, and M. T. Rowe. 1996. Inactivation of Mycobacterium paratuberculosis in cows' milk at pasteurization temperatures. Appl. Environ. Microbiol. 62:631-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grant, I. R., H. J. Ball, and M. T. Rowe. 1997. A novel staining technique for assessing clumping and viability of Mycobacterium paratuberculosis cells during pasteurization, p. 374-376. In R. J. Chiodini, M. E. Hines II, and M. T. Collins (ed.), Proceedings of the Fifth International Colloquium on Paratuberculosis. International Association for Paratuberculosis, Rehoboth, MA.
- 11.Griffiths, M. W. 2006. Mycobacterium paratuberculosis, p. 522-556. In Y. Motarjemi and M. Adams (ed.), Emerging foodborne pathogens. Woodhead Publishing Ltd., Cambridge, United Kingdom.
- 12.Guerrero-Beltrán, J. A., and G. V. Barbosa-Cánovas. 2004. Review: advantages and limitations on processing foods by UV light. Food Sci. Technol. Int. 10:137-147. [Google Scholar]
- 13.Hammer, P., C. Kiesner, H.-G. Walte, and P. Teufel. 2006. Inactivation of Mycobacterium avium subsp. paratuberculosis in whole milk, skim milk and cream in a pilot plant pasteurizer. Kiel. Milchwirtsh. Forschungsber. 58:17-40. [Google Scholar]
- 14.Hanes, D. E., R. W. Worobo, P. A. Orlandi, D. H. Burr, M. D. Miliotis, M. G. Robl, J. W. Bier, M. J. Arrowood, J. J. Churey, and G. J. Jackson. 2002. Inactivation of Cryptosporidium parvum oocysts in fresh apple cider by UV irradiation. Appl. Environ. Microbiol. 68:4168-4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris, N. B., and R. G. Barletta. 2001. Mycobacterium avium subsp. paratuberculosis in veterinary medicine. Clin. Microbiol. Rev. 14:489-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hermon-Taylor, J., and T. Bull. 2002. Crohn's disease caused by Mycobacterium avium subspecies paratuberculosis: a public health tragedy whose resolution is long overdue. J. Med. Microbiol. 51:3-6. [DOI] [PubMed] [Google Scholar]
- 17.LeChevallier, M. W., and K. K. Au. 2004. Water treatment and pathogen control: process efficiency in achieving safe drinking water. IWA Publishing and World Health Organization, London, United Kingdom.
- 18.Matak, K. E., J. J. Churey, R. W. Worobo, S. S. Sumner, E. Hovingh, C. R. Hackney, and M. D. Pierson. 2005. Efficacy of UV light for the reduction of Listeria monocytogenes in goat's milk. J. Food Prot. 68:2212-2216. [DOI] [PubMed] [Google Scholar]
- 19.Peccia, J., and M. Hernandez. 2004. UV-induced inactivation rates for airborne Mycobacterium bovis BCG. J. Occup. Environ. Hyg. 1:430-435. [DOI] [PubMed] [Google Scholar]
- 20.Quintero-Ramos, A., J. J. Churey, P. Hartman, J. Barnard, and R. W. Worobo. 2004. Modeling of Escherichia coli inactivation by UV irradiation at different pH values in apple cider. J. Food Prot. 67:1153-1156. [DOI] [PubMed] [Google Scholar]
- 21.Reinemann, D. J., P. Gouws, T. Cilliers, K. Houck, and J. R. Bishop. 2006. New methods for UV treatment of milk for improved food safety and product quality. ASABE paper no. 066088. American Society of Agricultural and Biological Engineers (ASABE), St. Joseph, MI.
- 22.Shama, G. 1999. Ultraviolet light, p. 2208-2214. In R. K. Robinson, C. Batt, and P. Patel (ed.), Encyclopedia of food microbiology, vol. 3. Academic Press, London, United Kingdom. [Google Scholar]
- 23.Stabel, J. R., E. M. Steadham, and C. A. Bolin. 1997. Heat inactivation of Mycobacterium paratuberculosis in raw milk: are current pasteurization conditions effective? Appl. Environ. Microbiol. 63:4975-4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stabel, J. R., and A. Lambertz. 2004. Efficacy of pasteurization conditions for the inactivation of Mycobacterium avium subsp. paratuberculosis in milk. J. Food Prot. 67:2719-2726. [DOI] [PubMed] [Google Scholar]
- 25.Stanley, E. 2005. Rapid detection of viable Mycobacterium paratuberculosis in milk samples. Ph.D. thesis. University of Nottingham, Nottingham, United Kingdom.
- 26.Stanley, E. C., R. J. Mole, R. J. Smith, S. M. Glenn, M. R. Barer, M. McGowan, and C. E. Rees. 2007. Development of a new rapid combined phage and PCR method for detection and identification of viable Mycobacterium paratuberculosis bacteria within 48 hours. Appl. Environ. Microbiol. 73:1851-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wright, J. R., S. S. Sumner, C. R. Hackney, M. D. Pierson, and B. W. Zoecklein. 2000. Efficacy of ultraviolet light for reducing Escherichia coli O157:H7 in unpasteurized apple cider. J. Food Prot. 63:563-567. [DOI] [PubMed] [Google Scholar]