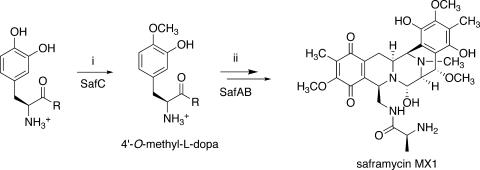
FIG. 1.
Proposed biosynthetic route to saframycin MX1. (i) SAM methylation. (ii) NRPS-catalyzed formation of a tetrapeptide intermediate. This intermediate is folded by a Pictet-Spengler or Bischler-Napieralski condensation into a saframycin precursor. Further methylation and oxidation lead to the formation of saframycin MX1. This study defines intermediate 4′-O-methyl-l-dopa as the first committed intermediate; previously, the timing of C- versus O-methylation was unclear.