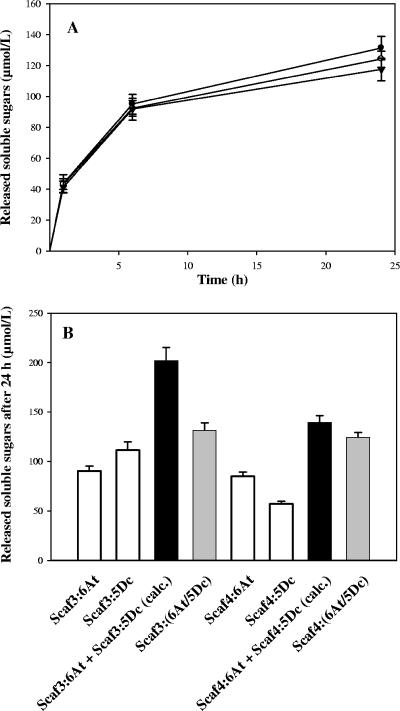
FIG. 8.
Hydrolysis of Avicel by the fungal enzyme pair 6At plus 5Dc in the free states and various complexed states. (A) Kinetics of Avicel hydrolysis by 6At and 5Dc in the free state or bound to Scaf3 or Scaf4. Curves are labeled as follows: free 6At plus 5Dc, ▾; Scaf3-(6At/5Dc), •; Scaf4-(6At/5Dc), ○. The data are the means of four independent experiments (variation within ±5%). (B) Avicelase activities of 6At plus 5Dc bound jointly or individually to Scaf3 and Scaf4. The designated proteins were mixed in stoichiometric amounts, and the status of the protein mixture was verified by gel filtration analysis (data not shown) prior to addition of the substrate. White bars, soluble sugars released by single-enzyme complexes; gray bars, soluble sugars released by Scaf3- and Scaf4-based complexes; black bars, calculated (calc.) sum of soluble sugars released by single-enzyme complexes individually. The data are the means of four independent experiments, and the bars indicate the standard deviations.