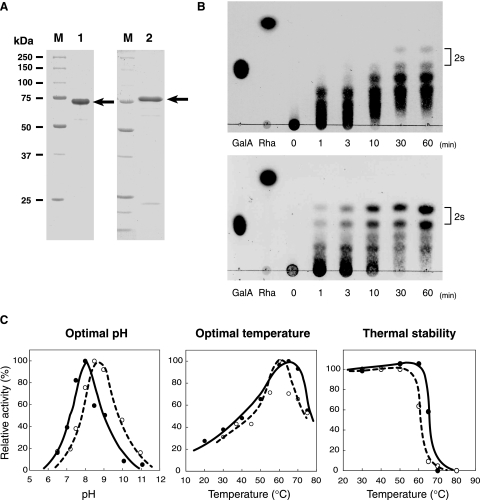
FIG. 3.
Properties of RG lyases. (A) SDS-PAGE of purified YesW and YesX followed by protein staining with Coomassie brilliant blue. Lane M contained molecular mass standards (from top to bottom)—synthetic polypeptides with molecular masses of 250, 150, 100, 75, 50, 37, and 25 kDa. Lane 1, purified recombinant YesW (5 μg protein); lane 2, purified recombinant YesX (5 μg protein). The arrow indicates the position of each enzyme. (B) Enzymatic degradation of RG-I. Degradation of the RG chain by YesW (upper) and YesX (lower). The reaction mixture consisted of 5 mg/ml of the RG chain, 50 mM of Tris-HCl (pH 7.5), 2 mM of CaCl2, and the purified enzyme. Reaction products were periodically sampled and analyzed on TLC plates, followed by staining with sulfuric acid. Reaction times (min) are indicated in the figure. The spots indicated by “2s” are unsaturated RG disaccharides with and without an addition of ammonium ion, which was used during preparation of the RG chain. Galacturonic acid (GalA) and rhamnose (Rha) were used for marker control. (C) Effects of pH and temperature on the activity and stability of YesW and YesX. The relative activity of YesW is indicated by a closed circle and solid line and that of YesX by an open circle and broken line. For optimal pH (left panel), activity was assayed with HEPES-NaOH (pH 6.5 and 7.2), Tris-HCl (pH 7.5, 8.0, and 8.5), and glycine-NaOH (pH 9.0, 10.0, and 11.0). Activity at pH 8.0 in YesW and pH 8.5 in YesX is taken as 100%. For optimal temperature (center panel), activity at 65°C in YesW and 60°C in YesX is taken as 100%. For thermal stability (right panel), purified enzymes were preincubated for 5 min at the temperatures indicated and residual enzyme activity was measured. The activity of enzymes preincubated at 4°C for 5 min is taken as 100%.