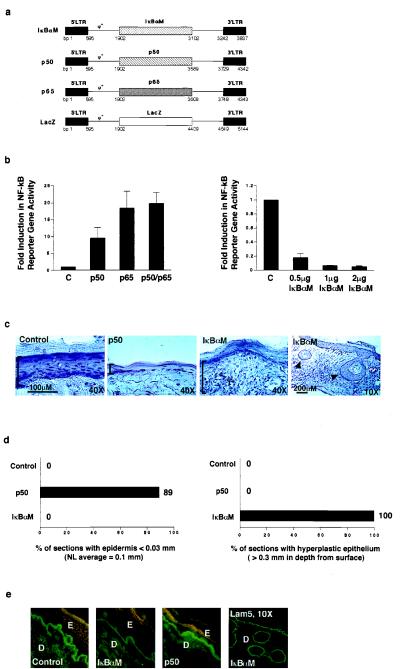
Figure 5.
Impact of NF-κB on human epithelial growth in vivo. (a) Retroviral expression vectors for proteins exerting inhibitory and activating effects on NF-κB function. Diagrammed are vectors for dominant-negative mutant IκBαM, constitutively nuclear p50 (p50), and p65. The LZRS lacZ vector (35) served as a control for these studies. (b) Impact of inhibitory and activating NF-κB proteins on NF-κB-driven gene expression in epithelial cells in vitro. The panel of vectors above was expressed in human keratinocytes along with a reporter construct containing three copies of NF-κB DNA consensus binding sites driving expression of the luciferase reporter gene. All transfections were performed in triplicate. Twenty-four hours following gene transfer, cell extracts were prepared and analyzed for reporter gene activity. Data are reported as fold induction in reporter gene activity, normalized for transfection efficiency by using a cotransfected Rous sarcoma virus–chloramphenicol acetyltransferase internal control. C, lacZ control. For assessment of IκBαM dominant-negative effects, NF-κB activity was induced for 4 h with 30 ng/ml of the phorbol ester phorbol 12-myristate 13-acetate prior to performance of reporter gene analysis. (c) Tissue architecture of human skin expressing either activating or inhibitory NF-κB subunits and lacZ control. Retroviral expression vectors for proteins activating or inhibiting NF-κB function were utilized to transduce primary human keratinocytes in vitro. These cells were then used to regenerate human skin on CB.17 scid/scid mice. Histologic appearance is shown; immunostaining of each tissue section with species-specific antibodies to human involucrin was used to confirm human tissue origin prior to hematoxylin/eosin staining. Brackets define epidermal thickness; arrows in the low power (×10) field of IκBαM skin denote focal areas of deep hyperplasia. (d) Frequency of histologic abnormalities in human epidermis in vivo. Multiple 5-μM sections were obtained in a stepwise fashion through tissue biopsies that spanned the full 1.5 cm thickness of each regenerated human graft. Atrophic changes were defined as less than 30% thickness of viable epidermis as compared with normal average value of 0.1 mm found in controls. Deep hyperplasia was defined as epithelial tissue penetrating underlying dermis to a depth of at least 0.3 mm. For p50, a total of 9 representative tissue sections were analyzed from all grafted mice; for IκBαM, n =12; and for lacZ and unengineered control, n = 6. The data are expressed as the percent of individual tissue sections displaying the given histologic abnormality. (e) Expression of involucrin in human skin expressing either activating or inhibitory NF-κB subunits and lacZ control. Double immunostaining was performed with human species-specific antibodies to involucrin and laminin 5, a basement membrane zone protein used to highlight the inferior boundary of the basal epidermal layer; involucrin (rhodamine), and laminin 5 (fluorescein isothiocyanate). Low power field of laminin 5 immunostained IκBαM[+] skin is also shown to highlight the boundaries of deep human epithelium. E, epidermis; D, dermis.