Abstract
Helicobacter pylori CagA is translocated into gastric epithelial cells by a type IV secretion system and interacts with the Src homology 2 phosphatase, altering cell morphology. Multiple EPIYA motifs in CagA are associated with increased activity in cells and with gastric cancer. The aim of this work was to study the heterogeneity in activity in cells of multiple H. pylori single colonies isolated from a single patient and its association with polymorphism in cagA. The presence of cagA, cagE, cagT, and cag10 was studied with 318 H. pylori isolates from the antra and corpora of 18 patients. AGS gastric epithelial cells were infected with 75 isolates, and interleukin-8 (IL-8) secretion, cytoskeletal changes, CagA translocation, and tyrosine phosphorylation were measured. The cagA 3′-variable region was sequenced for 30 isolates to determine the number and types of EPIYA motifs. Isolates from an individual stomach were usually genetically related and had quantitatively similar phenotypic effects on cells (IL-8 induction and cytoskeletal changes). However, strains from different patients with similar CagA EPIYA motif patterns varied widely in these phenotypes. Among isolates with an EPIYA-ABC pattern, the phenotype was variable: IL-8 induction ranged from 200 to 1,200 pg/ml, and morphological changes occurred in 20 to 70% of cells. In several cases, cagA sequence diversity appeared to explain the lack of CagA activity, as isolates with an EPIYA-ACC pattern or a modified B motif had reduced cell activity. cag pathogenicity island-positive H. pylori isolates displayed a high level of heterogeneity in the capacity to induce IL-8 secretion and morphological changes; an absent or modified B motif was associated with low activity.
Helicobacter pylori infects up to 50% of the world's human population, lives for decades in the human stomach (17), and is associated with duodenal ulcers (16), gastric ulcers, distal gastric adenocarcinoma, and gastric mucosa-associated lymphoid tissue lymphoma (13). Its ability to cause disease has been associated with the expression of several virulence factors, including the VacA cytotoxin (5, 6), BabA (19), the neutrophil-activating protein (HP-NAP) (30, 38), outer inflammatory protein A (OipA) (50), the duodenal ulcer-promoting gene (dupA) (28), and the cag pathogenicity island (cagPAI). The cagPAI is a 40-kb region originally acquired horizontally and inserted into the glutamate racemase gene (1, 15); it is present in about 50 to 60% of H. pylori isolates from Western countries and in >90% of isolates from East Asian countries. Some studies have shown that severe gastroduodenal diseases are associated with H. pylori strains that harbor an intact cagPAI (11, 22, 24, 26, 29, 32, 33), whereas other studies could not find a relationship (7, 25).
Genes in the cagPAI encode a type IV secretion system (17) through which CagA is translocated into gastric epithelial cells, where it is phosphorylated by Src kinases on the tyrosine residues of a five-amino-acid (EPIYA) motif (10, 34, 40, 44). Once phosphorylated CagA interacts with Src homology 2 (SHP-2) phosphatase, it stimulates downstream signaling cascades involved in the reorganization of the cytoskeleton, resulting in cellular morphological changes such as the “hummingbird” phenotype, which is characterized by a prominent elongation and spreading of host cells, including the production of filopodia and lamellipodia (20, 39). Attachment of H. pylori to gastric epithelial cells also induces the production of interleukin-8 (IL-8), mobilizing inflammatory cells to the site of infection in a process involving NF-κB activation (36, 42, 43). Recently, some authors reported that IL-8 release might be mediated by mitogen-activated protein kinases through tyrosine phosphorylation of CagA (14, 27).
The cagA gene contains a 5′ end which is highly conserved and a 3′ end which is variable. The 3′-variable region contains several repeat sequences, each of which contains an EPIYA motif; the size variation in CagA correlates with the number of repeat sequences located in this region (47). EPIYA motifs in Western H. pylori isolates are classified as EPIYA-A, EPIYA-B, and EPIYA-C and are defined by the amino acid sequences surrounding the EPIYA motifs. It has been suggested that the number of EPIYA-C sites directly correlates with the level of tyrosine phosphorylation, SHP-2 binding activity, and cell damage. CagA proteins in East Asian H. pylori isolates possess EPIYA-A, EPIYA-B, and EPIYA-D motifs; the EPIYA-D motif has a higher affinity for SHP-2 than does the Western EPIYA-C motif and appears to induce more severe cellular changes (21).
Evidence showing that CagA proteins with more EPIYA motifs are more frequent in strains associated with cases of atrophic gastritis and gastric cancer than in strains from patients with chronic gastritis has been presented, suggesting an association between the size of the 3′-variable region of cagA and the clinical outcome (8, 9). In South Africa, H. pylori strains with four to six EPIYA motifs and higher CagA molecular weights were isolated from patients with gastric cancer (4). In addition, the study demonstrated that when H. pylori is cocultured with gastric epithelial cells, a correlation between the size of the CagA protein, the magnitude of tyrosine phosphorylation, and the intensity of cellular elongation exists (4). Similar results have been reported for strains from Chinese patients (51).
Several studies have addressed the correlation between genetic diversity in the cagPAI and disease, but few have reported on phenotypic diversity regarding the cellular activities of multiple isolates obtained from single patients. Accordingly, the aim of this work was to study phenotypic diversity in the activity in gastric epithelial cells of multiple H. pylori single colonies isolated from single patients and to correlate this diversity with the polymorphisms observed in the 3′-variable region of the cagA gene.
(Part of this work was submitted as a requirement for the D.Sc. degree of Adriana Reyes-Leon at Doctorado en Ciencias Biomedicas, Universidad Nacional Autonoma de Mexico.)
MATERIALS AND METHODS
Patients.
We studied 10 children with chronic abdominal pain (seven boys and three girls) attending the Gastroenterology Department at the Hospital de Pediatria, Centro Medico Nacional Siglo XXI (CMN-SXXI), Instituto Mexicano del Seguro Social (IMSS), Mexico City, Mexico, with a mean age of 10.7 years (ranging from 7 to 16 years), and eight adults (five men and three women) attending the Gastroenterology Department at the Hospital de Especialidades, CMN-SXXI, IMSS, with a mean age of 57.2 years (ranging from 28 to 88 years). Three adult patients had gastric ulcers, four had duodenal ulcers, and one had nonulcer dyspepsia. Patients were subjected to endoscopy as part of the usual diagnostic protocol, and two biopsies each were taken from the antrum and corpus; one biopsy from each region was used to culture H. pylori. The study was approved by the ethics committee of the Hospital de Pediatria at CMN-SXXI, IMSS.
Helicobacter pylori culture and isolation of multiple single colonies from biopsies.
Antrum and corpus biopsy specimens were placed in sterile 0.9% saline solution, homogenized, and inoculated onto blood agar base (BBL, MD) plates supplemented with 5% sheep blood. The plates were incubated at 37°C in a 9% CO2 atmosphere for up to 5 days. H. pylori was identified by colony and microscopic morphology and by positive oxidase, catalase, and urease tests. From each primary growth, 7 to 10 single colonies each were isolated from the antrum and corpus and propagated on blood agar medium. We studied a total of 200 isolates from children (mean of 20 per patient) and 118 isolates from adults (mean of 15 per patient).
Detection of cagPAI genes.
DNAs were isolated from confluent plate cultures of each isolate, using the commercial Wizard method (Promega Corporation, Madison, WI) according to the manufacturer's instructions. DNAs from H. pylori strains 84-183 (ATCC 53726), 26695 (ATCC 700392), and Tx30a (ATCC 51932) were prepared for use as controls. The cagE, cagT, and cag10 genes were selected for study because they are distributed along the cagPAI and because they are homologous to known genes in type IV secretion systems; in addition, cagA was studied because it encodes the protein which is translocated by this system. The presence of these genes was evaluated in the 318 H. pylori isolates by PCRs using specific primers (Table 1). Two sets of primers were used for each gene examined, except for the cag10 gene (Table 1). All PCR mixtures consisted of 1 μl of chromosomal DNA template (100 ng), 1× PCR buffer, 1.5 mM MgCl2, a 0.2 mM concentration of each deoxynucleoside triphosphate (Boehringer Mannheim, Germany), 25 pmol of each primer, and 1.25 units of Taq DNA polymerase (Invitrogen, Life Technologies, Brazil) in a final volume of 25 μl. PCRs were performed in a thermal cycler (GeneAmp PCR system 9700; PE Applied Biosystems). Positive (strains 26695 and 84-183) and negative (strain Tx30a) controls for the cagPAI were included in each run. To test for the absence of the cagPAI, we used the cag empty-site assay with primers 2 and 25, which flank the left and right ends of the cagPAI (1). The PCR conditions for each reaction were described previously (1, 24, 29, 37, 47, 51). PCR products were electrophoresed, stained with ethidium bromide, and visualized under UV light.
TABLE 1.
Specific primers used to identify cagPAI genes in this study
| Gene | Primer | Primer sequence (5′→3′) | Product size (bp) | Reference |
|---|---|---|---|---|
| cagA (5′ end) | F1 | GATAACAGGCAAGCTTTTTGAGG | 349 | 47 |
| B1 | CTGCAAAAGATTGTTTGGCAG | 349 | 47 | |
| cagA (3′ end) | cag2 | GGAACCCTAGTCGGTAATG | 450-850 | 37 |
| cag4 | ATCTTTGAGCTTGTCTATCG | 450-850 | 37 | |
| cagE | 101 | TTGAAAACTTCAAGGATAGGATAGAGC | 510 | 48 |
| 102 | GCCTAGCGTAATATCACCATTACCC | 510 | 48 | |
| PBRT-F | AAGGGTAAAGAAATGGGACTG | 1,800 | 51 | |
| PBRT-R | GGAAGCGTGATAAAAGAGCAATGT | 1,800 | 51 | |
| cagT | cagT-F | ATGAAAGTGAGAGCAAGTGT | 823 | 29 |
| cagT-R | TCACTTACCACTGAGCAAAC | 823 | 29 | |
| CAG13 | TCTAAAAAGATTACGCTCATAGGCG | 489 | 24 | |
| CAG14 | CTTTGGCTTGCATGTTCAAGTTGCC | 489 | 24 | |
| cag10 | cag10-F | ATGGAAGACTTTTTGTATAA | 2,208 | 29 |
| cag10-R | TCACAGTTCGCTTGAACCCA | 2,208 | 29 | |
| Empty site | 2 | ACATTTTGGCTAAATAAACGCTG | 360 | 1 |
| 25 | TCATGCGAGCGGCGATGTG | 360 | 1 | |
| RAPD-PCR | 1254 | CCGCAGCCAA | 2 | |
| 1281 | AACGCGCAAC | 2 |
To confirm the PCR results, dot blot hybridization was performed as follows. The PCR products for the cagA, cagE, cagT, and cag10 genes were amplified with specific primer pairs F1 and B1, 101 and 102, cagT-F and cagT-R, and cag10-F and cag10-R, respectively, using chromosomal DNA from strain 84-183. PCR products were electrophoresed, and appropriate fragments (probes) were purified (Rapid Gel extraction system; Marligen Bioscience) and radioactively labeled with [α-32P]dCTP by random primer extension (Megaprime DNA labeling system kit; Amersham Pharmacia Biotech). Hybridization was performed using Hybond-N+ nylon membranes (Amersham Pharmacia Biotech), using 20 ng of genomic DNA per spot for each isolate. Membranes were incubated with the probes, washed, and processed for autoradiography with X-ray film (Kodak).
RAPD-PCR method.
Random amplified polymorphic DNA PCRs (RAPD-PCRs) were carried out in a 25-μl volume containing 20 ng of H. pylori genomic DNA, 2.5 μl of 10× PCR buffer, 3.5 mM MgCl2, 20 pmol of primer 1254 or 1281 (Table 1), a 0.25 mM concentration of each deoxynucleoside triphosphate (Boehringer Mannheim, Germany), and 1.2 units of Taq DNA polymerase (Invitrogen, Life Technologies, Brazil), as previously reported (2). An aliquot of 20 μl of PCR product was electrophoresed in a 2% agarose gel at 80 V, stained, and visualized.
Sequencing of the 3′-variable region of the cagA gene.
The 3′-variable region of cagA was amplified with primers cag2 and cag4 (37) (Table 1), and nucleotide sequencing was performed using the dideoxynucleotide chain termination method with a BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems) in an ABI PRISM 377 automated DNA sequencer (Applied Biosystems), as previously described (8). Nucleotide and derived amino acid sequences were analyzed and aligned with the Chromas (version 1.62; Technelysium) and DNAMAN (version 3.0; Lynnon BioSoft) programs.
Biological activities of H. pylori on gastric epithelial cells.
For translocation, phosphorylation, and IL-8 induction assays, the AGS cell line (ATCC CRL-1739) was grown in 75-cm2 flasks with Ham's F-12 nutrient mixture (GIBCO, Invitrogen Corporation) supplemented with 10% heat-inactivated fetal bovine serum (F-12-10% FBS) (GIBCO, Invitrogen Corporation, USA) at 37°C in a 5% CO2 atmosphere for 48 h. Next, serum-free F-12 medium was added, and cells were incubated for an additional 24 h. For cellular elongation assays, AGS cells (1 × 105/ml) were grown in six-well plates with F-12-10% FBS for 48 h. H. pylori strains to be tested were grown for 48 h in blood agar plates, and a single colony was reseeded on an agar plate and incubated for 24 h. H. pylori colonies were harvested and resuspended in serum-free F-12 medium to reach an optical density of 0.1 at 550 nm (1.2 × 108 bacteria/ml) before addition to AGS cells at a multiplicity of infection of 1:100. AGS cells in either 75-cm2 flasks or six-well plates were cocultured with H. pylori isolates in serum-free F-12 medium for up to 48 h (see below). H. pylori strains 26695 and 60190 (ATCC 49503) were used as positive controls.
After coculture for 6 h, the cell culture supernatant was collected and stored at −80°C until it was tested for IL-8 induction. The amount of IL-8 in each sample was measured by an enzyme-linked immunosorbent assay, using an OptEIA human IL-8 kit (BD Biosciences) following the manufacturer's instructions. Cells were then washed twice with phosphate-buffered saline (PBS) containing 1 mM calcium chloride and 0.5 mM magnesium chloride, scraped from the flasks into 5 ml PBS containing 1 mM sodium vanadate, harvested by centrifugation at 1,000 × g for 10 min, and resuspended in 100 μl PBS-sodium vanadate and 50 μl 4× sample loading buffer (0.2 M Tris-HCl, pH 6.8, 0.4 M dithiothreitol, 8% sodium dodecyl sulfate, 40% glycerol, 0.4% bromophenol blue). Samples were boiled and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Proteins were separated in 9% polyacrylamide gels, electrotransferred to nitrocellulose membranes by standard procedures, and examined for the presence of the CagA protein by using a polyclonal antibody (1:1,000) (bN-20; Santa Cruz Biotechnology). Phosphorylated tyrosine was detected with a monoclonal antibody (1:3,000) (PY99; Santa Cruz Biotechnology). Blots were then incubated with horseradish peroxidase-conjugated rabbit anti-goat and goat anti-mouse antibodies (Zymed Laboratories, CA) and developed with ECL Western blotting detection reagents (Amersham Biosciences, Piscataway, NJ). In some cases, translocated CagA was detected using sera from infected patients (1:50 dilution in PBS) with high levels of anti-CagA antibodies, incubated with a peroxidase-conjugated goat antibody to human immunoglobulin G (Zymed Laboratories, CA) (1:1,000 dilution), and developed with ECL Western blotting detection reagents (18).
For induction of cellular elongation of AGS cells, AGS cells infected with H. pylori isolates were incubated for up to 48 h. Cells were then examined for cellular elongation by light microscopy (magnification, ×20), reading three randomly chosen fields (4); results were reported as percentages of cells exhibiting the cellular elongation effect.
Each isolate was tested for IL-8 induction, CagA translocation and phosphorylation, and cellular elongation in two separate experiments, with each sample run in duplicate in each assay.
H. pylori adherence and viability during coinfection.
To assess H. pylori adherence to AGS cells, coinfections were monitored by light microscopy (×40) during different periods to visualize bacteria attached to cells and bacterial mobility. The viability of the inoculated bacteria was monitored 1, 24, and 48 h after coinfection; for this purpose, an aliquot of 10 μl of a 1:100 dilution of the cell culture supernatant was taken, spread on blood agar plates, and cultured for 48 h.
Statistical analysis.
A two-tailed Student t test was used to assess the relationship of cagPAI+ and cagPAI− strains to induction of IL-8 release and cellular elongation in AGS cells. A P value of <0.05 (two-sided) was considered statistically significant.
Nucleotide sequence accession numbers.
Nucleotide sequences were deposited in GenBank under accession numbers EF552407 to EF552424.
RESULTS
Presence of cagA, cagE, cagT and cag10 genes.
Among the 318 H. pylori isolates studied, 250 isolates (78.6%) had all four cagPAI genes (cagA, cagE, cagT, and cag10) (cagPAI+) and 48 isolates (15.1%), which were positive for the PCR empty site, did not have the cagPAI genes (cagPAI− isolates) (Table 2). In all 20 isolates from patient 365, cagA was absent, but cagE, cagT, and cag10 were present, indicating a partial deletion in the cagPAI. Patient 259 was colonized with both cagPAI+ and cagPAI− isolates, which had different RAPD patterns (data not shown), showing evidence of a mixed infection (Table 2).
TABLE 2.
Characteristics of H. pylori isolates tested for the presence of the cagPAI, the size of the cagA 3′ region, and activity on cells
| Patient group | Patient | Diagnosisa | No. of isolates | cagPAI statusb | Size of cagA 3′ region (bp) | No. of isolates used in AGS coculture expts/no. of isolates sequenced | EPIYA patternc |
|---|---|---|---|---|---|---|---|
| Children | 365 | CAP | 20 | Partial | 4/0 | ||
| 646 | CAP | 20 | + | 500 | 6/2 | ACC | |
| 525 | CAP | 20 | + | 550 | 3/2 | ABC | |
| 648 | CAP | 20 | + | 550 | 5/2 | AB&C | |
| 236 | CAP | 20 | + | 570 | 4/2 | ABC | |
| 291 | CAP | 20 | + | 570 | 5/1 | ABC | |
| 482 | DU | 20 | + | 570 | 3/2 | ABC | |
| 475 | CAP | 20 | + | 650 | 9/2 | ABCC | |
| 307 | CAP | 20 | + | 650 | 4/2 | AB&CC | |
| 555 | CAP | 19 | + | 550 | 3/2 | ABC | |
| 1 | + | 650 | 1/1 | ABCC | |||
| Adults | 251 | DU | 14 | − | − | 3/0 | − |
| 252 | GU | 14 | − | 2/0 | |||
| 254 | NUD | 17 | − | 2/0 | |||
| 248 | GU | 15 | + | 550 | 4/2 | AB*C | |
| 249 | DU | 15 | + | 800 | 2/2 | ABCCC | |
| 256 | DU | 16 | + | 850 | 4/2 | ABABC | |
| 259 | DU | 8 | + | 550 | 5/2 | ABC | |
| 2 | + | 500 | 2/2 | ABC | |||
| 3 | − | 2/0 | |||||
| 261 | GU | 13 | + | 550 | 1/1 | AB&C | |
| 1 | + | 800 | 1/1 | AB&AB&C | |||
| Total | 318 | 75/30 |
CAP, chronic abdominal pain; DU, duodenal ulcer; GU, gastric ulcer; NUD, nonulcer dyspepsia.
+, present; −, absent.
B* motif, EPTYAQVAKKV; B& motif, EPIYTQVAKKV.
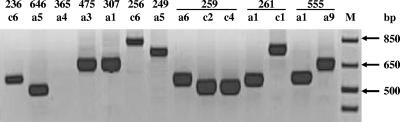
The 3′-variable region of the cagA gene was amplified from the 250 cagA-carrying isolates. There was diversity in the size of the fragment among isolates, with PCR products showing lengths of between 500 and 850 bp (Table 2). Although in the majority of cases all isolates from a patient showed the same length, in three cases (patients 555, 259, and 261) isolates presented different sizes for the cagA 3′-variable region. For patient 555, 19 isolates had a cagA fragment size of 550 bp and one had a size of 650 bp. Patient 261 had one isolate with a cagA fragment of 800 bp, whereas all others had a size of 550 bp (Table 2 and Fig. 1); analysis by RAPD-PCR showed that this variation was due to infection with multiple H. pylori strains. For patient 259, most isolates had a fragment size of 550 bp and two isolates had a size of 500 bp (Table 2 and Fig. 1); RAPD-PCR analysis showed that these isolates were of the same strain, with polymorphic variation in the 3′ region.
FIG. 1.
Determination of the size of the cagA 3′-variable region by PCR. PCR products from single-colony isolates from different patients or from the same patient (259, 261, and 555) showed size variation. Lane M, molecular size marker.
Diversity in IL-8 secretion by AGS cells cocultured with cagPAI+ and cagPAI− isolates.
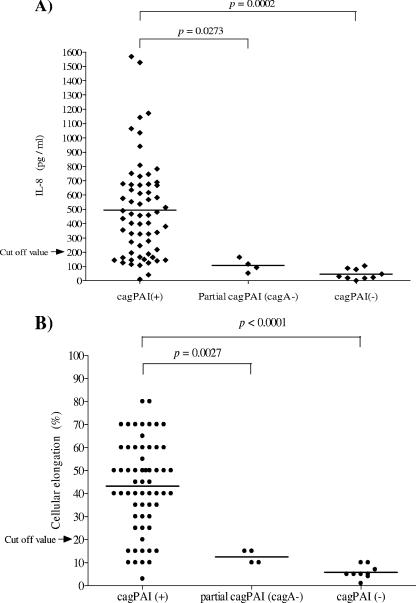
We selected a group of 75 H. pylori isolates, 62 of which were cagPAI+, 4 of which had a partial cagPAI (cagA negative), and 9 of which were cagPAI−, to analyze their ability to induce IL-8 secretion (Fig. 2A). Among the cagPAI+ H. pylori isolates tested, wide diversity in the ability to induce IL-8 secretion was observed, with IL-8 levels which ranged from <50 to over 1,500 pg/ml. IL-8 levels induced by cagPAI− isolates and by isolates with a partial cagPAI (cagA negative) were below 200 pg/ml in all cases, which is significantly lower than levels induced by cagPAI+ isolates (Fig. 2A).
FIG. 2.
Diversity in biological activities on AGS cells of 62 cagPAI+ isolates, 4 isolates with a partial cagPAI (cagA negative), and 9 cagPAI− isolates. (A) Induction of IL-8 expression. (B) Induction of cellular elongation. The intensities of both activities varied considerably among the cagPAI+ isolates and were significantly higher than those of partial cagPAI and cagPAI− isolates. Horizontal lines indicate mean values. The results represent the means for three independent experiments. The cutoff values for both activities were estimated as the means plus 3 standard deviations of the values obtained with the Tx30a reference strain and nine cagPAI− isolates.
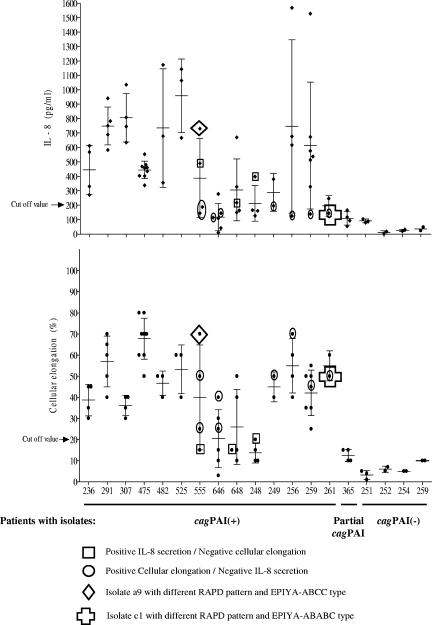
Isolates from individual patients, on the whole, gave similar results (Fig. 3). However, wide variation was seen between patients. For further analysis, we developed a definition of a non-IL-8-inducing isolate; using a cutoff value of the mean plus 3 standard deviations of the values obtained with the Tx30a reference strain and the nine cagPAI− isolates (<200 pg/ml), 16 of the 62 cagPAI+ isolates were defined as negative for IL-8 induction (25.8%). Among these 16 isolates, 5 isolates were from patient 646 (6 isolates), 3 were from patient 248 (4 isolates), and a fraction of isolates were from patients 648, 555, 259, 256, 249, and 261 (Fig. 3).
FIG. 3.
Diversity in the induction of cellular elongation and IL-8 secretion by multiple cagPAI+ H. pylori strains, isolates with a partial cagPAI, and cagPAI− isolates from single patients. Patient numbers are indicated on the x axis, and intensities of cell activities are shown on the y axes. Horizontal lines indicate mean values, and bars indicate standard deviations. The results represent the means for three independent experiments.
Diversity in the induction of cellular elongation in AGS cells cocultured with cagPAI+ and cagPAI− isolates.
AGS cells were infected with the same 75 H. pylori isolates that were tested for IL-8 induction. Among the H. pylori cagPAI+ isolates tested, there was wide diversity in the ability to induce cellular elongation, with values ranging from <10% to over 70% of cells affected. cagPAI− isolates and isolates with a partial cagPAI (cagA negative) induced elongation in <20% of the cells (Fig. 2B); these values were significantly lower than those induced by cagPAI+ isolates. In general, isolates from the same patient induced similar values, although wide variation was seen between patients. Most strains inducing high levels of IL-8 also caused the most cytoskeletal changes, although there were clear exceptions (e.g., patients 307, 475, 249, and 261)(Fig. 3). We defined the cutoff value for cellular elongation as the mean plus 3 standard deviations of the values obtained with the Tx30a reference strain (negative control) and nine cagPAI− isolates (<20%). Using this cutoff value, 11 of the 62 (17.7%) cagPAI+ isolates were negative for cellular elongation, with 4 of 4 isolates from patient 248 and a fraction of isolates from patients 648, 646, and 555 being negative (Fig. 3).
H. pylori adherence and viability during coinfection.
cagPAI+ isolates with low or high activity on AGS cells were monitored for adherence and viability. Both types of isolates, either positive or negative for cell activity, showed similar patterns of adherence to AGS cells, which increased over time, as shown in Fig. S1 in the supplemental material; the mobility of bacteria was evident during the 48 h of the assay. Viable H. pylori cells (>1010 CFU/ml) could be recovered on agar plates even after 48 h of coinfection with both types of isolates; in addition, images show the growth of H. pylori colonies on the surfaces of AGS cells after hours of coinfection (see Fig. S1 in the supplemental material). In summary, no difference was observed in adherence, mobility, or viability between isolates with low or high activity on cells.
Translocation and phosphorylation of CagA in AGS cells.
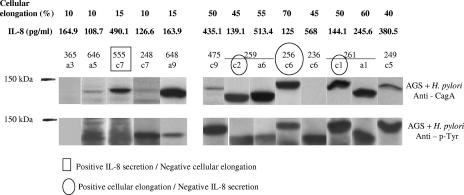
The above results showed that H. pylori cagPAI+ isolates had variable effects on AGS cells. To further study this variability, we analyzed the translocation and phosphorylation of the CagA protein in the same 75 isolates in AGS cells. We observed differences in the size of the CagA proteins, which correlated with variation in the size of the 3′-variable region of cagA (Fig. 4). According to our results, 58 of the 62 cagPAI+ isolates tested had CagA translocated and phosphorylated within AGS cells. In the remaining four isolates (all from patient 236), the CagA protein was not recognized by the commercial polyclonal anti-CagA antibody (bN-20) we used; however, the translocated CagA protein was recognized by the anti-phosphorylated tyrosine antibody (PY99) (Fig. 4). In addition, the CagA proteins from these isolates were recognized by immunoglobulin G in sera from two H. pylori-infected patients (see Fig. S2 in the supplemental material).
FIG. 4.
Translocation and phosphorylation of the CagA protein in AGS cells infected with cagPAI+ and partial cagPAI-carrying H. pylori isolates. Western blot analysis of CagA translocation into AGS cells was performed with a polyclonal anti-CagA antibody, and detection of CagA phosphorylation was done with an anti-phosphotyrosine monoclonal antibody. Isolate 365a3 is a partial cagPAI-carrying (cagA negative) isolate; all other isolates (from 646a5 to 249c5) are cagPAI+. Isolate 236c6 was negative for Western blotting with anti-CagA but positive with anti-phospho-Tyr. Isolates with variability in biological activity levels are marked with circles or squares.
As expected, the isolates with a partial cagPAI, with absence of the cagA gene, and the cagPAI− isolates did not express the CagA protein and did not have phosphorylated CagA in AGS cells. Of interest, the cagPAI+ isolates that were noninducers of either cellular elongation or IL-8 secretion were still able to translocate and phosphorylate CagA in AGS cells, although in several cases (such as isolate 648a9) phosphorylation was weak (Fig. 4).
Diversity in the biological activities of multiple H. pylori isolates from single patients.
Heterogeneity in the activities of multiple isolates from single patients on AGS cells was analyzed. Although most isolates from single patients were rather homogeneous with regard to cell activity (Fig. 3), significant diversity was observed in some cases. Isolates from patients 555, 256, and 259 displayed a high level of diversity in both cellular elongation and IL-8 induction. In addition, we identified isolates causing a strong cellular elongation phenotype which were negative for IL-8 induction (from patients 555, 646, 249, 256, 259, and 261). Isolates negative for cellular elongation but positive for IL-8 induction (from patients 555, 648, and 248) were also found, although in all these cases induction of IL-8 was rather weak (Fig. 3).
Sequencing of the 3′-variable region of the cagA gene.
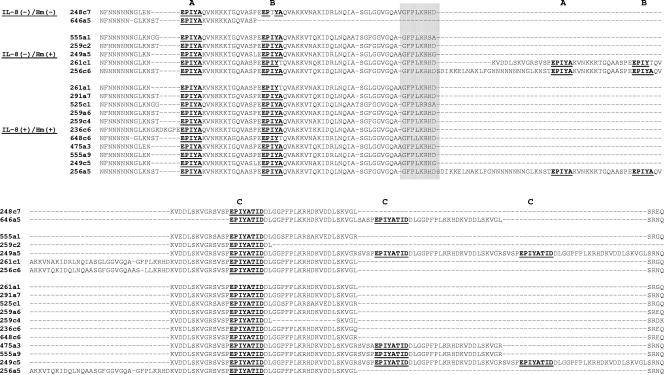
From the 62 cagPAI+ H. pylori isolates tested in the above studies, we chose 30 isolates for sequencing, representing the different sizes in the 3′ region of the cagA gene and different levels of activity on AGS cells (Table 2). For all 30 of these isolates, cagA had the Western type sequence (Fig. 5). The number of EPIYA motifs varied among these isolates; 20 isolates (66.6%) had three motifs, 5 (16.7%) had four motifs, and 5 (16.7%) had five motifs. The majority of the isolates with three EPIYA motifs were of the ABC type, and only two isolates (from patient 646) were of the ACC type, with a deletion between the A and C motifs. All 20 of these isolates had a 3′ cagA size of ≤570 bp (Table 2). The isolates with four EPIYA motifs were of the ABCC type, and all had a 3′ cagA size of 650 bp. Among the five isolates with five EPIYA motifs, two were of the ABCCC type and three were of the ABABC type, and they had 3′ cagA sizes of between 800 and 850 bp (Table 2).
FIG. 5.
Deduced amino acid sequences of C-terminal repeat regions of CagA from cagPAI+ isolates that were positive or negative for induction of IL-8 or cellular elongation (Hm). The EPIYA-A, -B, and -C motifs and the SHP-2 binding site after the EPIYA-C motif are underlined, and the Western sequence type is shaded.
Isolates from patient 259 yielded 3′ cagA PCR products of 500 bp (isolates c2 and c4) and 550 bp (isolates a1 and a6); these PCR products were sequenced. Compared with isolates a1 and a6, isolate c2 had a deletion of ∼24 amino acids after the EPIYA-C motif (Fig. 5); of interest, another isolate from the corpus, c4, also showed a deletion after the EPIYA-C motif, although this was shorter (∼15 amino acids). All isolates from this patient showed similar RAPD patterns, suggesting that they were the same strain. However, isolate c2 was negative for IL-8 induction, while isolates a1, a6, and c4 were positive, suggesting that the long deletion after the EPIYA-C motif might be associated with a loss of biological activity. Among the 20 isolates tested from patient 555, 19 had a 3′ cagA size of 550 bp and an ABC pattern, whereas one had a size of 650 bp (a9) and an ABCC pattern. Isolate a9 displayed higher levels of IL-8 secretion and cellular elongation than did isolates with the ABC pattern; isolate a9 had a different RAPD pattern, implying that it was a different coinfecting strain. Fourteen isolates were tested from patient 261; 13 had a 3′ cagA size of 550 bp and an ABC pattern, whereas one had a size of 800 bp (c1) and an ABABC pattern. IL-8 secretion and cellular elongation levels were lower in the isolate with the ABABC pattern. Isolate c1 also had a different RAPD pattern (data not shown), implying that it was a different coinfecting strain (Fig. 3). Thus, of three patients with isolates differing in the size of the 3′ cagA region, two had mixed infections and one had the same strain with polymorphism in the 3′ sequence.
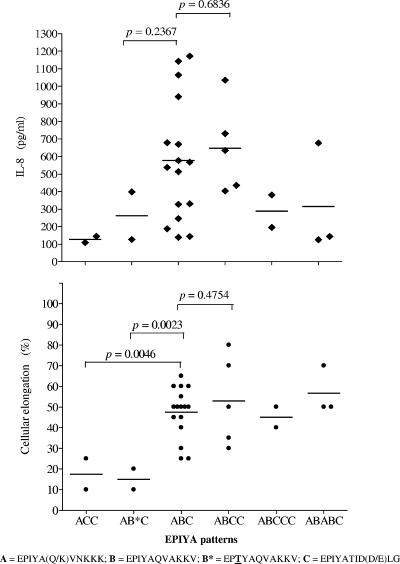
Association of the sequence in the 3′ region of cagA with activity on AGS cells.
We next analyzed the sequence of the 3′-variable region, searching for a possible association with activity on cells, especially for isolates which lacked activity on AGS cells. There was no clear association between the number and type of EPIYA motifs and the ability to induce IL-8 secretion or cellular elongation (Fig. 6). However, several interesting results were found in the sequences for isolates negative for both cellular elongation and IL-8 induction; some had an EPIYA-ACC pattern (patient 646) (Fig. 5), suggesting the importance of the EPIYA-B motif for cell activity. Isolates from patient 248 with an EPIYA-ABC pattern had a modified B motif (B* [EPTYA]); three of four isolates were negative for IL-8 induction, and all four were negative for induction of cellular elongation (Fig. 6). Isolates from patient 307 with an EPIYA-ABCC pattern had a different modification of the B motif (B& [EPIYT]) and induced lower levels of cellular elongation and IL-8 secretion than did isolates with the normal ABCC pattern (Fig. 6).
FIG. 6.
Relationship between EPIYA patterns and the ability to induce both IL-8 secretion and cellular elongation for 30 cagPAI+ H. pylori isolates from our population. Isolates without the B motif (ACC pattern; patient 646) or with a modified B motif (EPTYA; patient 248) had a significantly weaker cellular elongation phenotype.
For isolates from patient 236 that were cagA positive but whose protein products were not recognized by the commercial anti-CagA polyclonal antibody (Fig. 1), we observed a short insertion of five amino acids (DKGPE) before the EPIYA-A motif which was not observed for isolates from any other patient (Fig. 5); of interest, isolates from this patient caused moderate IL-8 and cellular elongation induction (Fig. 3).
DISCUSSION
cagA is a highly polymorphic gene with diversity in its 3′-end region, which is important for the biological activity of the encoded protein on gastric epithelial cells. This region encodes the tyrosine phosphorylation site and the SHP-2 phosphatase binding site. The interaction of these proteins activates a series of signaling pathways, causing an increase in proliferation and abnormal cell motility (45, 46). It is therefore important to extend studies on polymorphism in this 3′ region of cagA across populations and to correlate this diversity with the biological activities of strains. In the present study, we addressed both the extent of polymorphism in the cagA gene and the diversity in the biological activities of Mexican strains on gastric epithelial cells.
To better describe the extent of polymorphisms, we chose to study multiple single colonies isolated from different sites of the stomach of individual patients. All isolates from the antra and corpora of 13 of the 18 patients studied had a homogeneous cagPAI content, one had a partial cagPAI content (patient 365), and for only one patient was a mixed infection with cagPAI+ and cagPAI− strains documented (patient 259). However, in spite of the rather homogeneous gene content in the cagPAI, diversity in the sequence of the cagA 3′ region was observed in isolates from three patients. In one case, the multiple isolates were found to be of the same strain (patient 259), and in two cases, the strains were not the same as those documented by RAPD analysis (patients 555 and 261); thus, mixed infection was documented in two cases and polymorphism in the 3′ region was shown for one patient.
Once we had determined that most isolates in this study were positive for the cagPAI, we next analyzed phenotypic diversity by studying the heterogeneity in the activities of these isolates on epithelial cells. cagPAI− isolates and isolates with a partial cagPAI (cagA negative) induced very low levels of cellular elongation (<20%) and IL-8 (<200 pg/ml), as expected. On the other hand, even among isolates positive for the four genes of cagPAI tested, including cagA, there were a number of isolates which were unable to induce IL-8 secretion or cellular elongation, which suggests that the mere presence of the cagPAI is not sufficient for these activities. In several of these cases, low phosphorylation of CagA might explain the low cell activity, but in others negative activity was observed in spite of the translocation and phosphorylation of CagA. It should be stressed that in all cases, the CagA protein was translocated and phosphorylated, suggesting the presence of a functional type IV secretion system. Polymorphisms in the 3′ cagA region explained many of these cases and illustrate the importance of the EPIYA-B motif; thus, strains lacking this motif (ACC pattern) were unable to induce either IL-8 secretion or cellular elongation, an observation which has not been reported previously. In addition, strains with a modified B motif (EPTYA) also displayed weak activity, which further confirms the importance of the whole motif for cell activity. Deletions after the EPIYA-C motif were also associated with either a low or absent biological activity. These results show that negative or weak IL-8 or cellular elongation induction in cagPAI+ isolates might be due to specific modifications in the CagA sequence. In accordance with these results, Brandt et al. recently reported that among cagPAI+ strains there are high and low IL-8 inducers, and this variability was associated with the number of EPIYA motifs and the amino acid residues surrounding these motifs, stressing the need for CagA to induce IL-8 in epithelial cells (14). The diversity in the activities of different cagPAI+ H. pylori isolates on AGS cells does not appear to be influenced by the degree of adherence of the isolates to AGS cells or their ability to grow during the coinfection experiment, since we found similar adherence and growth on AGS cells by H. pylori isolates with either high or low cell activity.
Of note, among the cagPAI+ isolates, the range of IL-8 induction varied as much as 30 times (from 50 to over 1,500 pg/ml), and the intensity of induction of cellular elongation varied over 15 times (from 5 to 80%); such wide diversity has not been documented previously, and as discussed below, polymorphisms in the cagA gene partially explain this diversity. Higashi et al. described that the variation in biological effects of CagA is caused by the variation in the number and sequence of tyrosine phosphorylation sites (21). This correlation was not observed in our study, as exemplified by the fact that IL-8 and cellular elongation activities of isolates with an ABC pattern varied from no to high induction. The amount of CagA translocated and phosphorylated had a correlation with low activity in several isolates but not in others. As discussed above, in some of these cases variations in the sequence of the B motif might explain the observed low activity on cells. Still, when cellular elongation was analyzed, the degree of induction tended to increase in the direction ACC→AB*C→ABC→ABCC→ABABC, which partially agrees with previous reports (4, 31). This correlation was not observed for IL-8 induction, which is also in agreement with previous reports (3, 4, 35) and confirms that although the role of CagA in both activities is controversial, the mechanism of action is different for each activity (41). This is further supported by the fact that isolates from six patients displayed a moderate or strong cellular elongation activity but no IL-8 induction. Some reports have proposed a key role for cagE in IL-8 induction (35); however, our results disagree with this, as all isolates with negative or low IL-8 induction had the cagE gene present. Still, we cannot discard the possibility that like the case for cagA, sequence diversity in cagE might cause differences in IL-8 induction. Other authors have also reported that H. pylori can induce NF-κB activation (and IL-8 secretion), via Nod1, in epithelial cells that respond to peptidoglycan delivered to the cytoplasm of the cell by the type IV secretion system encoded by the cagPAI (49).
When phenotypic diversity was analyzed in multiple isolates from a single patient, with all isolates representing unique strains, in many cases a rather homogeneous degree of activity was documented among the isolates. However, there were patients for whom the activities of the isolates on cells varied over 10 times. Some of these cases might be explained by polymorphisms in the cagA sequence among the colonizing isolates; sequence diversity, insertions, or deletions were documented in such cases. However, in other cases, such diversity cannot be explained by heterogeneity in the cagA sequence, and polymorphisms in other genes of the cagPAI or outside the island might help to explain this diversity. It should be noted that isolates of a single patient classified as the same isolate with tests such as RAPD or amplified fragment length polymorphism analysis may still show variation in the gene content of 3 to 5% of the genome (12, 23), and this variation may cause phenotypic diversity. The mechanisms responsible for the observed diversity in these patients deserve further study.
An interesting case was patient 236, whose H. pylori strain produced a CagA protein which could not be recognized by the commercial antibody used in this study; sequence studies showed that it had a five-amino-acid insertion before the EPIYA-A motif. Conceivably, this might cause changes in the structure of the protein sufficient to avoid recognition by particular antibodies. However, this modified protein was still recognized by serum from the same patient from whom the strain was isolated as well as by sera from other patients.
In conclusion, our study documents a high level of diversity in the ability of cagPAI+ strains to induce both IL-8 production and cellular elongation in epithelial cells; such diversity might be explained partially by polymorphisms in the 3′ region of cagA. Another novel observation was the finding that isolates lacking the EPIYA-B motif (pattern ACC) or with a modified B motif displayed a negative or weak activity on epithelial cells; to our knowledge, such a possible association has not been reported previously. We documented important diversity in the activities of isolates from single patients on cells, which in some, but not all, cases could potentially be explained by polymorphisms in cagA.
Supplementary Material
Acknowledgments
This work was partially supported by CONACYT, Mexico (Sectorial Salud; grant CO1-6957). J.T. is a recipient of a Fundacion IMSS exclusivity scholarship.
Editor: F. C. Fang
Footnotes
Published ahead of print on 16 April 2007.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Akopyants, N., S. W. Clifton, D. Kersulyte, J. E. Crabtree, B. E. Youree, C. A. Reece, N. O. Bukanov, E. S. Drazek, B. A. Roe, and D. Berg. 1998. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol. Microbiol. 28:37-53. [DOI] [PubMed] [Google Scholar]
- 2.Akopyanz, N., N. O. Bukanov, T. U. Westblom, S. Kresovich, and D. E. Berg. 1992. DNA diversity among clinical isolates of Helicobacter pylori detected by PCR-based RAPD fingerprinting. Nucleic Acids Res. 20:5137-5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aras, R. A., Y. Lee, S. K. Kim, D. Israel, R. M. Peek, Jr., and M. J. Blaser. 2003. Natural variation in populations of persistently colonizing bacteria affect human host cell phenotype. J. Infect. Dis. 188:486-496. [DOI] [PubMed] [Google Scholar]
- 4.Argent, R. H., M. Kidd, R. J. Owen, R. J. Thomas, M. C. Limb, and J. C. Atherton. 2004. Determinants and consequences of different levels of CagA phosphorylation for clinical isolates of Helicobacter pylori. Gastroenterology 127:514-523. [DOI] [PubMed] [Google Scholar]
- 5.Atherton, J. C., P. Cao, R. M. Peek, Jr., M. K. Tummuru, M. J. Blaser, and T. L. Cover. 1995. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J. Biol. Chem. 270:17771-17777. [DOI] [PubMed] [Google Scholar]
- 6.Atherton, J. C., R. M. Peek, Jr., K. T. Tham, T. L. Cover, and M. J. Blaser. 1997. Clinical and pathological importance of heterogeneity in vacA the vacuolating cytotoxin gene of Helicobacter pylori. Gastroenterology 112:92-99. [DOI] [PubMed] [Google Scholar]
- 7.Audibert, C., C. Burucoa, B. Janvier, and J. L. Fauchére. 2001. Implication of the structure of the Helicobacter pylori cag pathogenicity island in induction of interleukin-8 secretion. Infect. Immun. 69:1625-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azuma, T., A. Yamakawa, S. Yamazaki, K. Fukuta, M. Ohtani, Y. Ito, M. Dojo, Y. Yamazaki, and M. Kuriyama. 2002. Correlation between variation of the 3′ region of the cagA gene in Helicobacter pylori and disease outcome in Japan. J. Infect. Dis. 186:1621-1630. [DOI] [PubMed] [Google Scholar]
- 9.Azuma, T., S. Yamazaki, A. Yamakawa, M. Ohtani, A. Muramatsu, H. Suto, Y. Ito, M. Dojo, Y. Yamazaki, M. Kuriyama, Y. Keida, H. Higashi, and M. Hatakeyama. 2004. Association between diversity in the Src homology 2 domain-containing tyrosine phosphatase binding site of Helicobacter pylori CagA protein and gastric atrophy and cancer. J. Infect. Dis. 189:820-827. [DOI] [PubMed] [Google Scholar]
- 10.Backert, S., S. Moese, M. Selbach, V. Brinkmann, and T. F. Meyer. 2001. Phosphorylation of tyrosine 972 of the Helicobacter pylori CagA protein is essential for induction of a scattering phenotype in gastric epithelial cells. Mol. Microbiol. 42:631-644. [DOI] [PubMed] [Google Scholar]
- 11.Backert, S., T. Schwarz, S. Miehlke, C. Kirsch, C. Sommer, T. Kwok, M. Gerhard, U. B. Goebel, N. Lehn, W. Koenig, and T. F. Meyer. 2004. Functional analysis of the cag pathogenicity island in Helicobacter pylori isolates from patients with gastritis, peptic ulcer and gastric cancer. Infect. Immun. 72:1043-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Björkholm, B., A. Lundin, A. Sillén, K. Guillemin, N. Salama, C. Rubio, J. I. Gordon, P. Falk, and L. Engstrand. 2001. Comparison of genetic divergence and fitness between two subclones of Helicobacter pylori. Infect. Immun. 69:7832-7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blaser, M. J., G. I. Perez-Perez, H. Kleanthous, T. L. Cover, M. Peek, P. Chyou, G. N. Stemmermann, and A. Nomura. 1995. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 55:2111-2115. [PubMed] [Google Scholar]
- 14.Brandt, S., T. Kwok, R. Harting, W. König, and S. Backert. 2005. NF-κB activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc. Natl. Acad. Sci. USA 102:9300-9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Censini, S., C. Lange, Z. Xiang, J. E. Crabtree, P. Ghiara, M. Borodovsky, R. Rappuoli, and A. Covacci. 1996. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. USA 93:14648-14653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Covacci, A., S. Censini, M. Bugnoli, R. Petracca, D. Burroni, G. Macchia, A. Massone, E. Papini, Z. Xiang, N. Figura, and R. Rappuoli. 1993. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc. Natl. Acad. Sci. USA 90:5791-5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Covacci, A., J. L. Telford, G. Del Giudice, J. Parsonnet, and R. Rappuoli. 1999. Helicobacter pylori virulence and genetic geography. Science 284:1328-1333. [DOI] [PubMed] [Google Scholar]
- 18.Eck, M., B. Schmauber, R. Haas, A. Greiner, S. Czub, and H. K. Müller-Hermelink. 1997. MALT-type lymphoma of the stomach is associated with Helicobacter pylori strains expressing the CagA protein. Gastroenterology 112:1482-1486. [DOI] [PubMed] [Google Scholar]
- 19.Gerhard, M., N. Lehn, N. Neumayer, T. Boren, R. Rad, W. Schepp, S. Miehlke, M. Classen, and C. Prinz. 1999. Clinical relevance of the Helicobacter pylori gene for blood-group antigen-binding adhesion. Proc. Natl. Acad. Sci. USA 96:12778-12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higashi, H., R. Tsutsumi, S. Muto, T. Sugiyama, T. Azuma, M. Asaka, and M. Hatakeyama. 2002. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science 295:683-686. [DOI] [PubMed] [Google Scholar]
- 21.Higashi, H., R. Tsutsumi, A. Fujita, S. Yamazaki, M. Asaka, T. Azuma, and M. Hatakeyama. 2002. Biological activity of the Helicobacter pylori virulence factor CagA is determined by variation in the tyrosine phosphorylation sites. Proc. Natl. Acad. Sci. USA 99:14428-14433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikenoue, T., S. Maeda, K. Ogura, M. Akanuma, Y. Mitsuno, Y. Imai, H. Yoshida, Y. Shiratori, and M. Omata. 2001. Determination of Helicobacter pylori virulence by simple gene analysis of the cag pathogenicity island. Clin. Diagn. Lab. Immunol. 8:181-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Israel, D. A., N. Salama, U. Krishna, U. M. Rieger, J. C. Atherton, S. Falkow, and R. M. Peek, Jr. 2001. Helicobacter pylori genetic diversity within the gastric niche of a single human host. Proc. Natl. Acad. Sci. USA 98:14625-14630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenks, P., F. Megraud, and A. Labigne. 1998. Clinical outcome after infection with Helicobacter pylori does not appear to be reliably predicted by the presence of any of the genes of the cag pathogenicity island. Gut 43:752-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kauser, F., A. A. Khan, M. A. Hussain, I. M. Carroll, N. Ahmad, S. Tiwari, Y. Shouche, B. Das, M. Alam, S. M. Ali, C. M. Habibullah, R. Sierra, F. Megraud, and L. A. Sechi. 2004. The cag pathogenicity island of Helicobacter pylori is disrupted in the majority of patient isolates from different human populations. J. Clin. Microbiol. 42:5302-5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kidd, M., A. J. Lastovica, J. C. Atherton, and J. A. Louw. 2001. Conservation of the cag pathogenicity island is associated with vacA alleles and gastroduodenal disease in South African Helicobacter pylori isolates. Gut 49:11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim, S., Y. Lee, H. Kim, and M. J. Blaser. 2006. Helicobacter pylori CagA transfection of gastric epithelial cells induces interleukin-8. Cell. Microbiol. 8:97-106. [DOI] [PubMed] [Google Scholar]
- 28.Lu, H., P. I. Hsu, D. Graham, and Y. Yamaoka. 2005. Duodenal ulcer promoting gene of Helicobacter pylori. Gastroenterology 128:833-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maeda, S., H. Yoshida, T. Ikenoue, K. Ogura, F. Kanai, N. Kato, Y. Shiratori, and M. Omata. 1999. Structure of cag pathogenicity island in Japanese Helicobacter pylori isolates. Gut 44:336-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montecucco, C., and M. de Bernard. 2003. Molecular and cellular mechanisms of action of the vacuolating cytotoxin (VacA) and neutrophil activating protein (HP-NAP) virulence factors of Helicobacter pylori. Microb. Infect. 5:715-721. [DOI] [PubMed] [Google Scholar]
- 31.Naito, M., T. Yamazaki, R. Tsutsumi, H. Higashi, K. Onoe, S. Yamazaki, T. Azuma, and M. Hatakeyama. 2006. Influence of EPIYA-repeat polymorphism on the phosphorylation dependent biological activity of Helicobacter pylori CagA. Gastroenterology 130:1181-1190. [DOI] [PubMed] [Google Scholar]
- 32.Nilsson, C., A. Sillén, L. Eriksson, M. Strand, H. Enroth, S. Normark, P. Falk, and L. Engstrand. 2003. Correlation between cag pathogenicity island composition and Helicobacter pylori-associated gastroduodenal disease. Infect. Immun. 71:6573-6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Occhialini, A., A. Marais, M. Urdaci, R. Sierra, N. Muñoz, A. Covacci, and F. Megraud. 2001. Composition and gene expression of the cag pathogenicity island in Helicobacter pylori strains isolated from gastric carcinoma and gastritis patients in Costa Rica. Infect. Immun. 69:1902-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Odenbreit, S., J. Püls, B. Sedlmaier, E. Gerland, W. Fischer, and R. Haas. 2000. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 287:1497-1500. [DOI] [PubMed] [Google Scholar]
- 35.Owen, R. J., S. I. Sharp, A. J. Lawson, Z. Durrani, S. Rijpkema, and M. Kidd. 2003. Investigation of the biological relevance of Helicobacter pylori cagE locus diversity, presence of CagA tyrosine phosphorylation motifs and vacuolating cytotoxin genotype on IL-8 induction in gastric epithelial cells. FEMS Immunol. Med. Microbiol. 36:135-140. [DOI] [PubMed] [Google Scholar]
- 36.Rieder, G., R. A. Hatz, A. P. Moran, A. Walz, M. Stolte, and G. Enders. 1997. Role of adherence in interleukin-8 induction in Helicobacter pylori-associated gastritis. Infect. Immun. 65:3622-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rudi, J., C. Kolb, M. Maiwald, D. Kuck, A. Sieg, P. R. Galle, and W. Stremmel. 1998. Diversity of Helicobacter pylori vacA and cagA genes and relationship to VacA and CagA protein expression, cytotoxin production, and associated diseases. J. Clin. Microbiol. 36:944-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Satin, B., G. Del Giudice, V. D. Bianca, S. Dusi, C. Laudanna, F. Tonello, D. Kelleher, R. Rappuoli, C. Montecucco, and F. Rossi. 2000. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a protective antigen and a major virulence factor. J. Exp. Med. 191:1467-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Segal, E., J. Cha, J. Lo, S. Falkow, and L. S. Tompkins. 1999. Altered states: involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc. Natl. Acad. Sci. USA 96:14559-14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Selbach, M., S. Moese, C. R. Hauck, T. F. Meyer, and S. Backert. 2002. Src is the kinase of the Helicobacter pylori CagA protein in vitro and in vivo. J. Biol. Chem. 277:6775-6778. [DOI] [PubMed] [Google Scholar]
- 41.Selbach, M., S. Moese, T. F. Meyer, and S. Backert. 2002. Functional analysis of the Helicobacter pylori cag pathogenicity island reveals both VirD4-CagA-dependent and VirD4-CagA-independent mechanisms. Infect. Immun. 70:665-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharma, S. A., M. K. Tummuru, G. G. Miller, and M. J. Blaser. 1995. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect. Immun. 63:1681-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma, S. A., M. K. Tummuru, M. J. Blaser, and L. D. Kerr. 1998. Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-κB in gastric epithelial cells. J. Immunol. 160:2401-2407. [PubMed] [Google Scholar]
- 44.Stein, M., F. Bagnoli, R. Halenbeck, R. Rappuoli, W. J. Fantl, and A. Covacci. 2002. c-Src/Lyn kinases activate Helicobacter pylori CagA through tyrosine phosphorylation of the EPIYA motifs. Mol. Microbiol. 43:971-980. [DOI] [PubMed] [Google Scholar]
- 45.Tsutsumi, R., H. Higashi, M. Higuchi, M. Okada, and M. Hatakeyama. 2003. Attenuation of Helicobacter pylori CagA-SHP-2 signaling by interaction between CagA and C-terminal Src kinase. J. Biol. Chem. 278:3664-3670. [DOI] [PubMed] [Google Scholar]
- 46.Tsutsumi, R., A. Takahashi, T. Azuma, H. Higashi, and M. Hatakeyama. 2006. Focal adhesion kinase is a substrate and downstream effector of SHP-2 complexed with Helicobacter pylori CagA. Mol. Cell. Biol. 26:261-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tummuru, M., T. L. Cover, and M. J. Blaser. 1993. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect. Immun. 61:1799-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tummuru, M., S. A. Sharma, and M. J. Blaser. 1995. Helicobacter pylori picB, a homologue of the Bordetella pertussis toxin secretion protein, is required for induction of IL-8 in gastric epithelial cells. Mol. Microbiol. 18:867-876. [DOI] [PubMed] [Google Scholar]
- 49.Viala, J., C. Chaput, I. G. Boneca, A. Cardona, S. E. Girardin, A. P. Moran, R. Athman, S. Memet, M. R. Huerre, A. J. Coyle, P. S. DiStefano, P. J. Sansonetti, A. Labigne, J. Bertin, D. J. Philpott, and R. L. Ferrero. 2004. Nod1 responds to peptidoglycan delivered by Helicobacter pylori cag pathogenicity island. Nat. Immunol. 5:1166-1174. [DOI] [PubMed] [Google Scholar]
- 50.Yamaoka, Y., D. H. Kwon, and D. Graham. 2000. A Mr 34,000 proinflammatory outer membrane protein (OipA) of Helicobacter pylori. Proc. Natl. Acad. Sci. USA 97:7533-7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang, Y., R. H. Argent, D. P. Letley, R. J. Thomas, and J. C. Atherton. 2005. Tyrosine phosphorylation of CagA from Chinese Helicobacter pylori isolates in AGS gastric epithelial cells. J. Clin. Microbiol. 43:786-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.