FIG. 7.
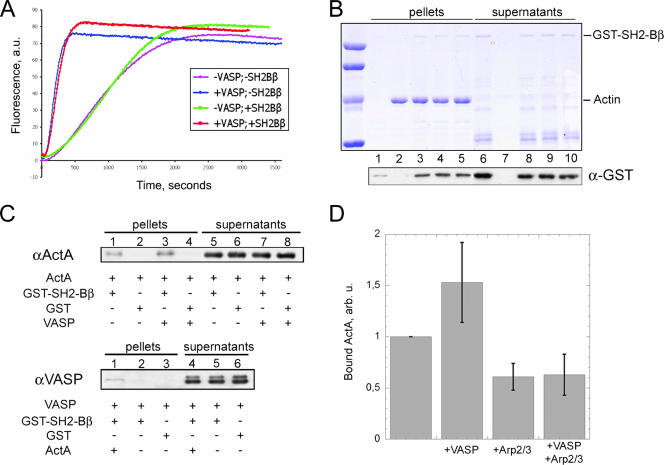
Interaction of SH2-Bβ with actin and ActA. (A) Actin (3 μM; 10% pyrenyl labeled) was polymerized in the presence of ActA-coated beads and the Arp2/3 complex (20 nM), with or without VASP (107 nM) and with or without SH2-Bβ (0.5 μM). Polymerization was monitored by the increase in fluorescence intensity of pyrenyl-actin. a.u., arbitrary units. (B) Actin was polymerized in the presence of SH2-Bβ and additional proteins. F-actin and G-actin were separated by high-speed centrifugation, and the proteins present in pellets (lanes 1 to 5) and supernatants (lanes 6 to 10) were detected by SDS-PAGE (top). Additional bands in the supernatants (lanes 6 and 8 to 10) are degraded forms of SH2-Bβ that do not bind F-actin, confirming that specific binding is observed in lanes 3 to 5. Immunoblotting using anti-GST antibody was done to detect SH2-Bβ with a higher contrast (bottom). Actin was polymerized alone (lanes 2 and 7), in the presence of 0.5 μM SH2-Bβ (lanes 3 and 8), or supplemented with 50 nM Arp2/3 complex and 40 nM ActA, without (lanes 4 and 9) or with (lanes 5 and 10) 50 nM VASP. The control without actin (lanes 1 and 6) shows a slight contamination (<5%) of the pellet by SH2-Bβ that is negligible compared to the amount of SH2-Bβ (∼30%) recruited by F-actin. (C) Pull-down assays of ActA and VASP with SH2-Bβ. (Top) ActA binds to GST-SH2-Bβ in the absence (lane 1) and, more strongly, in the presence (lane 3) of VASP. No ActA was detected in the negative controls (GST alone) (lanes 2 and 4). (Bottom) VASP alone (lane 2) does not bind to GST-SH2-Bβ beads and is recruited only via its strong interaction with ActA (lane 1). (D) Binding of ActA to SH2-Bβ as a function of Arp2/3 and VASP. In each experiment, the amount of ActA bound to GST-SH2-Bβ in the absence of other ligands is taken as a reference and normalized to 1 (left bar). The addition of VASP increased the amount of bound ActA by 50%. The addition of Arp2/3 induced a decrease of ∼40%, with or without VASP. Bars represent means plus SD, computed from three independent experiments.