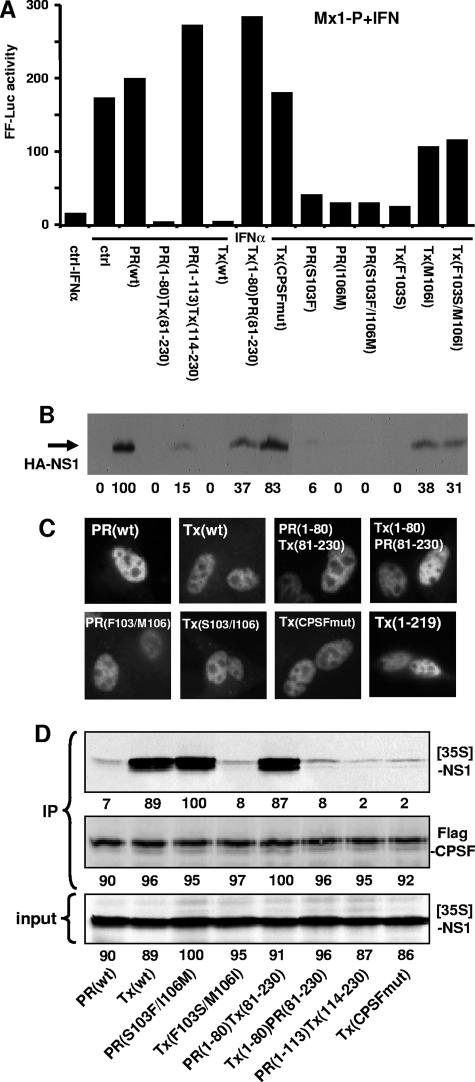
FIG. 3.
Identification of NS1 domains responsible for inhibition of gene expression and CPSF binding. (A) Vero cells were cotransfected with reporter plasmids carrying the FF-Luc gene under the control of the Mx1 promoter and the REN-Luc gene under the control of the SV40 promoter, together with expression plasmids encoding HA-tagged versions of NS1 of A/PR/8/34 and A/Tx/36/91 or with empty plasmid (ctrl). At 16 h posttransfection, the cells were treated with 200 U/ml of IFN-α2a for 16 h and then analyzed for reporter gene expression. (B) Lysates of transfected cells were analyzed for NS1 protein expression by Western blotting using an HA-specific antiserum. The numbers indicate the relative intensities of the NS1 protein bands, with PR NS1 activity defined as 100. (C) Analysis of subcellular accumulation of NS1 proteins. MDCK cells were transfected with the NS1 expression plasmids for 48 h. The cells were then fixed and analyzed by immunofluorescence, using a polyclonal antibody directed against the HA tag. (D) Analysis of CPSF binding. The HA-tagged NS1 constructs were expressed by in vitro translation in the presence of [35S]methionine (input). Flag-tagged CPSF was expressed in transfected 293T cells. The cell lysate was mixed with the 35S-labeled NS1 proteins and subjected to coimmunoprecipitation (IP) using a monoclonal anti-Flag antibody coupled to protein A-Sepharose. The beads were washed three times, and the precipitated proteins were analyzed for the presence of 35S-labeled NS1 by autoradiography and for Flag-CPSF by Western blot analysis using a Flag-specific rabbit antiserum. The numbers indicate the relative intensities of the protein bands, with the strongest signal defined as 100.