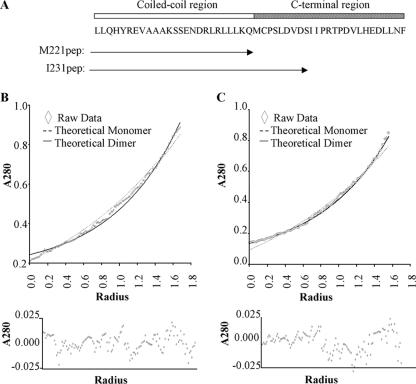
FIG. 3.
Stability of dimers revealed by analytical ultracentrifugation. (A) Schematic representation of residues contained within the two synthetic peptides and their locations compared to Zta structure. Representative analytical ultracentrifuge data sets for M221pep (B) and I231pep (C), recorded at 50,000 rpm, are shown and provide curves typical of sedimented and equilibrated species. Protein concentration (measured as A280) versus distance (r) from the center of the centrifuge rotor. The starting protein concentration was 500 μM. Experimental data points are shown for sedimentation of the peptide (diamonds). Also shown are simulated curves calculated assuming a completely monomeric (broken line) or dimeric (solid line) protein. The residual signals, calculated as the difference between the experimental and fitted data for a single ideal species, show no systematic errors, indicating that the fits are robust (lower panels).