Figure 1.
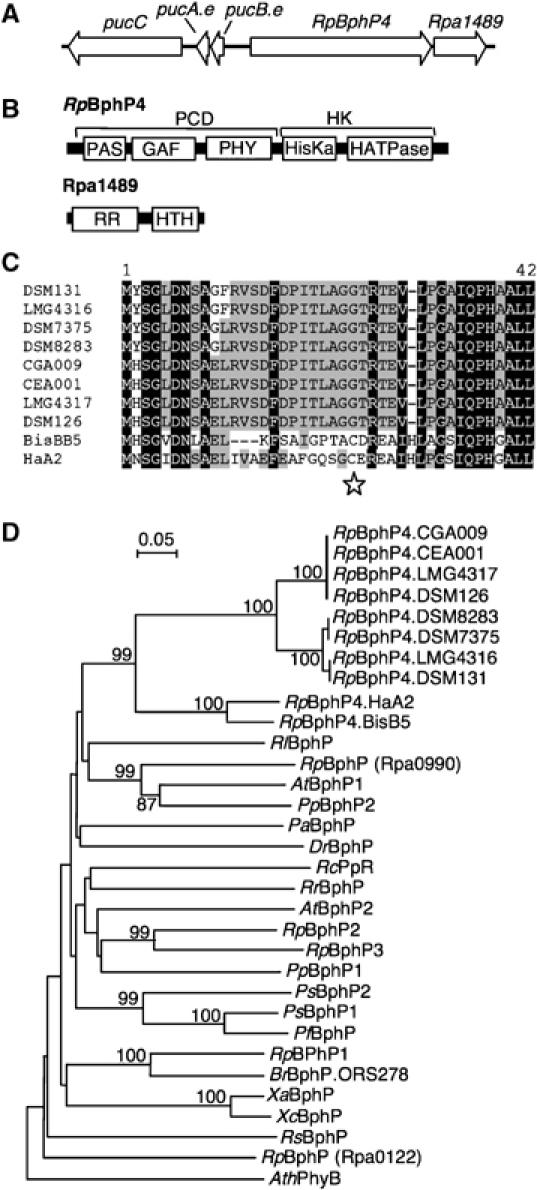
Molecular characterization of RpBphP4. (A) Arrangement of genes around RpBphP4 (rpa1490 from CGA009). (B) Predicted domain structure of RpBphP4 and Rpa1489. PCD: photosensory core domain; HK: histidine kinase domain; HisKa: phosphoacceptor domain; HATPase: ATP binding domain; RR: response regulator domain; HTH: helix–turn–helix domain. (C) AA sequence alignment of the N-termini of RpBphP4 proteins from different Rps. palustris strains. Residues conserved at more than 50 and 90% are highlighted in gray and black, respectively. Star indicates the Cys residue used as BV binding site. (D) Phylogenetic analysis of the BphP family based on an alignment of the GAF domains. The sequences were aligned by CLUSTALX and the tree was generated by the neighbor-joining method and displayed using NJPLOT. Bootstrap values, expressed as percentages of 1000 replications, are shown at the branching points. Species abbreviations: At, Agrobacterium tumefaciens; Ath, Arabidopsis thaliana; Br, Bradyrhizobium sp.; Dr, Deinococcus radiodurans; Pa, Pseudomonas aeruginosa; Pf, Pseudomonas fluorescens; Pp, Pseudomonas putida; Ps, Pseudomonas syringae; Rc, Rhodospirillum centenum; Rl, Rhizobium leguminosarum; Rp, Rhodopseudomonas palustris; Rr, Rhodospirillum rubrum; Rs, Rhodobacter sphaeroides; Xa, Xanthomonas axonopodis; Xc, Xanthomonas campestris.
