Figure 4.
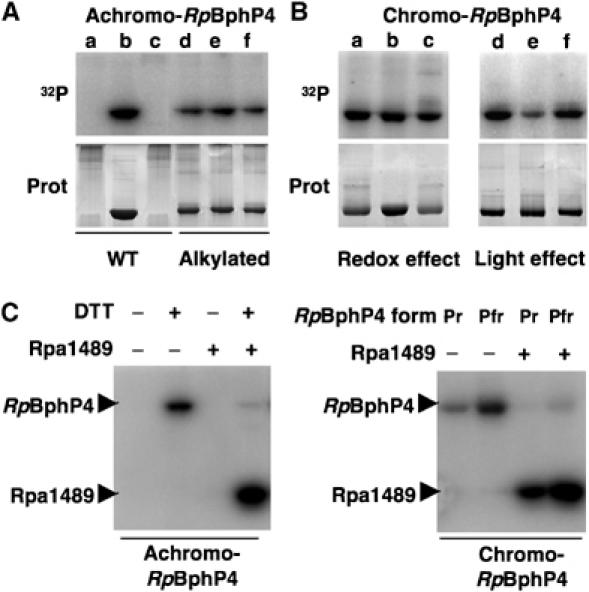
Achromo- and chromo-RpBphP4 proteins act as redox or light-regulated histidine kinases, respectively. (A) Effect of redox conditions on the kinase activity of achromo-RpBphP4 (WT) and alkylated achromo-RpBphP4 (alkylated). Lanes a and d, untreated; lanes b and e, reduced with 1 mM DTT; and lanes c and f, oxidized with 1 mM ferricyanide. Proteins were incubated with γ-32P-ATP for 15 min. The reaction products were separated by SDS–PAGE and the gels were autoradiographed (top) or stained with Coomassie blue (bottom). (B) Effect of redox or light conditions on the kinase activity of chromo-RpBphP4. For the redox effect, the sample was subjected to 15 min of pre-illumination with 705 nm light followed by 15 min of dark adaptation, and was left untreated (lane a), or treated with 1 mM DTT (lane b) or 1 mM ferricyanide (lane c). For the light effect, chromo-RpBphP4 was converted to its Pfr (lane d), Pr (lane e) or ‘meta R' (lane f) forms; the Pfr form was obtained by 15 min of pre-illumination at 705 nm followed by 15 min of dark adaptation, the Pr form by illumination at 770 nm and the ‘meta R' form by illumination at 705 nm. (C) Phosphotransfer between RpBphP4 and the RR Rpa1489. Transfer of the phosphoryl group between the achromo-RpBphP4 and Rpa1489 relative to the redox conditions (left panel). Phosphotransfer between chromo-RpBphP4 and Rpa1489 relative to the light conditions (right panel).
