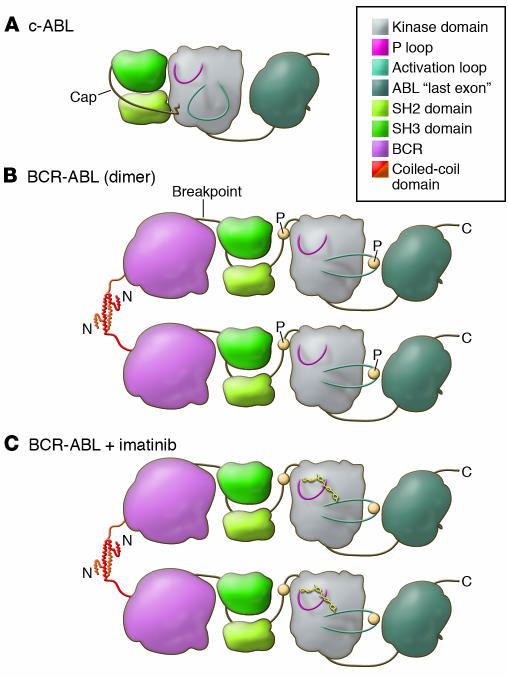
Figure 2. A model of the domains of the c-ABL and BCR-ABL proteins displays the functional consequences of the translocation.
(A) In c-ABL, the P-loop and the activation loop surround the active site for substrate phosphorylation (shaded). Under normal conditions c-ABL is inactive, with the SH2 and SH3 domains bound to the kinase domain, restricting its kinase activity (53). This conformation is stabilized by the cap domain, which is anchored to the kinase domain by a myristoyl group (jagged line) (52, 53). (B) In BCR-ABL, the myristoyl group of the cap domain is lost and is replaced by BCR, shown for simplicity as a single globular domain. This is presumed to destabilize binding of SH2 and SH3 to the kinase domain (56). The N terminus of BCR, the coiled-coil domain, forms a helical dimer (that also tetramerizes) and tethers individual BCR-ABL proteins together (54). This allows for transphosphorylation (P; yellow circles) of the kinase, which promotes the kinase to adopt an active conformation with a significant outward twist of the activation loop. (C) Imatinib binds to the active site of the kinase domain, freezing it in the inactive conformation, preventing BCR-ABL activation (25).