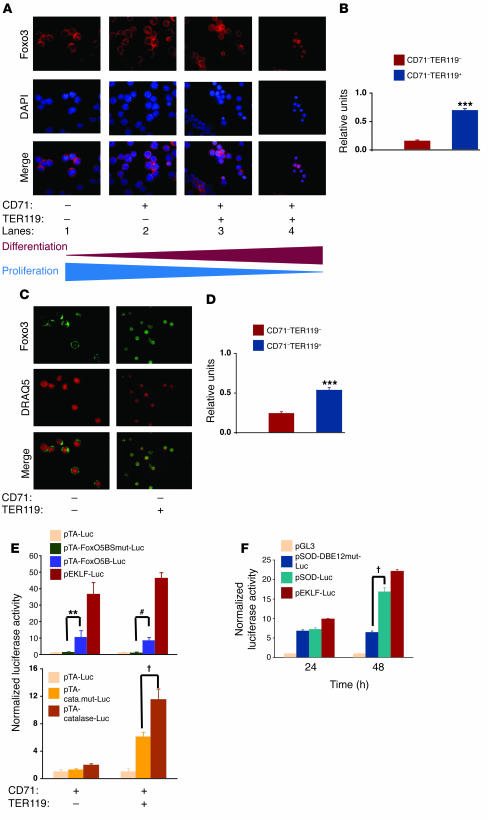
Figure 5. Foxo3 translocates to the nucleus and regulates transcription of anti-oxidant enzymes in primary fetal liver erythroblasts.
(A) Immunofluorescence staining of Foxo3 (red) in freshly isolated E14.5 fetal liver erythroid subpopulations using anti-FOXO3a antibody was performed and samples were counterstained with nuclear DAPI (blue). (B) Quantification of nuclear Foxo3 in CD71–TER119– cells as compared with CD71–TER119+ cells. Data were analyzed from an average of 50 cells from each subpopulation in A. (C) Foxo3 expression was investigated in nucleated, deep red fluorescing agent (DRAQ5) positive cells. DRAQ5+CD71–TER119– and DRAQ5+CD71–TER119+ cells were FACS sorted from E14.5 fetal liver and subjected to immunofluorescence staining using anti-FOXO3a antibody (green). (D) Quantification of results from C is shown for at least 50 cells from each subpopulation as mean ± SEM. ***P < 0.001, Student’s t test. (E) FACS-sorted erythroid precursor subpopulations CD71+TER119– and CD71+TER119+ cells were transfected with synthetic reporter containing 5 tandem repeat FoxO binding site (pTA-FoxO5BS-Luc) or mutant (pTA-FoxO5BSmut-Luc) (top panel), or a catalase luciferase reporter containing 2 FoxO binding sites (pTA-cata.mut-Luc) or mutant (pTA-catalase-Luc) (bottom panel), then cultured as previously described in the presence of Epo (2 U/ml) (28, 39), and luciferase activity was analyzed after 36 hours. EKLF reporter (pEKLF-Luc) (56) was used as a positive control. Data representative of normalized results from at least 4 independent experiments performed in triplicate are shown as mean ± SEM. **P < 0.01; †P < 0.002; #P < 0.0001. (F) Transcriptional activity of Foxo3 during maturation of fetal liver erythroid precursors. TER119– fetal liver cells (enriched in progenitors) were transiently transfected with an empty luciferase reporter (pGL-3) or a SOD2 luciferase reporter containing 2 FoxO binding sites (pSod-Luc) or its mutant (pSOD-DBE12mut-Luc) and cultured as described above, and luciferase activity was determined at 24 and 48 hours. Results are shown as mean ± SEM; n = 4 for each group.