Figure 4.
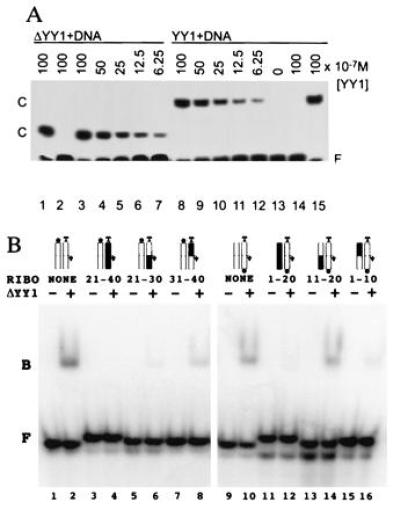
Nucleic acid binding properties of YY1 and ΔYY1. (A) YY1 and ΔYY1 have the same apparent affinity for the P5 initiator element, as judged by gel mobility shift assays. Reactions contained the same amount of radiolabeled crystallization DNA. The amount of YY1 and ΔYY1 added to each reaction is given above the lanes, and the positions of the protein–DNA complexes (C) are indicated. Lanes 2 and 14 include nonradioactive specific DNA competitor, and lanes 1 and 15 include nonradioactive nonspecific DNA competitor. (B) Binding of ΔYY1 to DNA/RNA hybrid analogs of the AAV P5 initiator element. The double-stranded synthetic oligonucleotides, radiolabeled at the 5′ end of the template (lanes 1–8) or nontemplate (lanes 9–16) strand were incubated with (even lanes) or without (odd lanes) ΔYY1 and analyzed as in A. The ribonucleotide substituted regions and schematic representations of the double-stranded oligonucleotides are shown above the lanes (RNA is shown in black with the dominant start site denoted by an arrow, template strand is labeled “T” and the radioactive phosphate is represented by a black dot). The positions of the free probes (F) and the protein-nucleic acid complexes (B) are indicated. Note: Direct comparisons of the binding reactions containing probe labeled on the nontemplate strand (lanes 1–8) to binding reactions containing probe labeled on the template strand (lanes 9–16) are not possible because the probes had different specific radioactivity. Each binding reaction contains equivalent amounts of ΔYY1 (0.5 pmol) and radioactive probe (0.1 pmol).
