Figure 1.
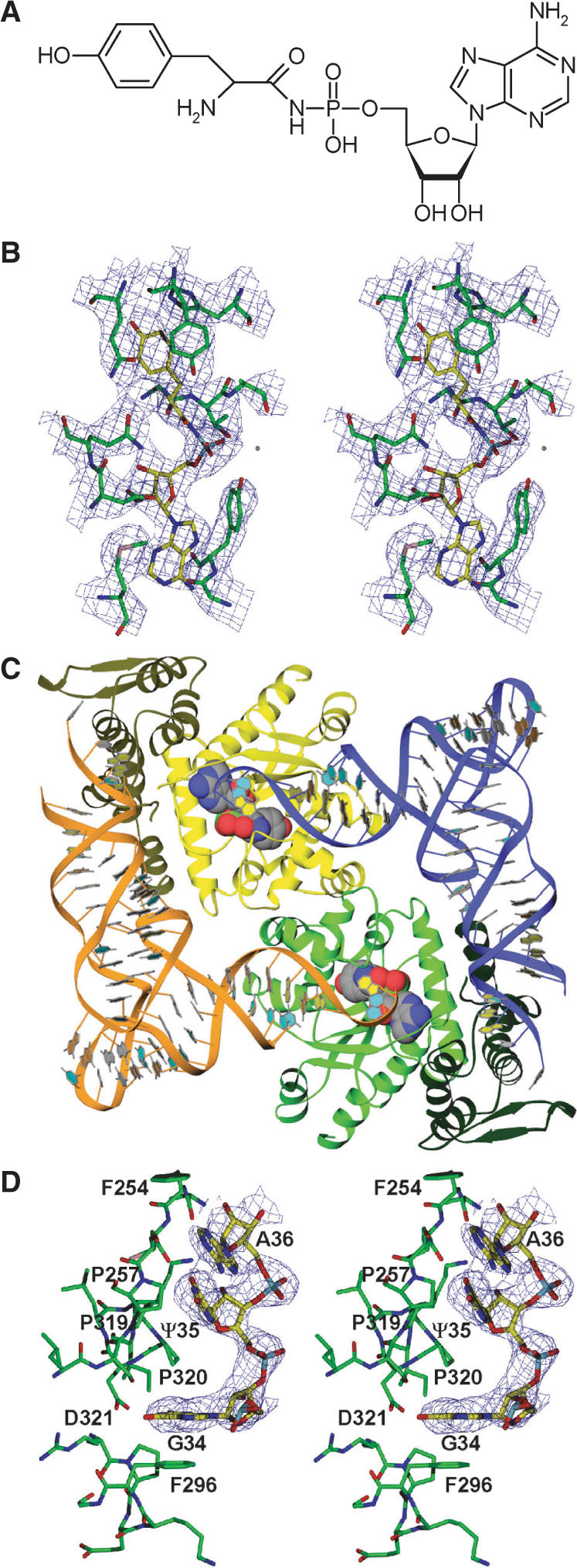
Structure determination of the ternary complex of SceTyrRS. (A) Structure of the Tyr-AMP analog (Tyr-AMPN), having an N-acyl phosphoramidate linkage where the oxygen atom of the mixed anhydride bond (-C-O-P-) of Tyr-AMP was replaced by an amino group (-C-NH-P-), used for the present crystal structure analysis. (B) Stereo diagram of the experimentally phased MAD map at 3.0-Å resolution of the Tyr-AMPN binding site of the ternary complex of SceTyrRS. The contour level is 1.2σ. (C) Ribbon drawing of the dimer of the ternary complex of SceTyrRS. The SceTyrRS (yellow and green) and tRNATyr (blue and orange) molecules are shown as ribbon models. The catalytic and anticodon-binding domains of TyrRS are shown as light and dark colors, respectively. The bound Tyr-AMPN molecules are shown as CPK models. The molecular two-fold axis [coinciding with the (1 1 0) crystallographic two-fold axis] is perpendicular to the plane of the paper. The structural discontinuity in the anticodon-loop of tRNATyr is due to the disordered nucleotides C32–U33. (D) Stereo diagram of the experimentally phased MAD map at 3.0-Å resolution showing the well-ordered anticodon bases (G34-Ψ35-A36) of Sce-tRNATyr. The contour level is 1.0σ.
