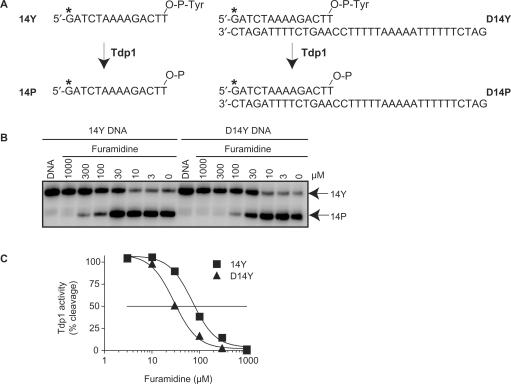
Figure 3.
Inhibition of Tdp1 activity by furamidine. (A) Schematic representation of the Tdp1 biochemical assays. The partially duplex oligopeptide D14Y or single-stranded 14Y were used as substrates. 32P-Radiolabeling (*) was at the 5′ terminus of the 14-mer strand. Tdp1 catalyzes the hydrolysis of the 3′-phosphotyrosine bond and converts 14Y and D14Y to an oligonucleotide with 3′-phosphate, 14P or D14P, respectively. (B) Representative gel showing Tdp1 inhibition by furamidine with single strand (14Y) and partially duplex (D14Y) substrates. Reactions were performed at 25°C for 20 min. Arrows indicate the 3′-phosphate oligonucleotide product (14P) that runs quicker than the corresponding tyrosyl oligonucleotide substrate (14Y) in a denaturing PAGE (18). The duplex D14Y substrate and D14P product are detected on the gel by their corresponding labeled single strands (14Y and 14P), as they are no longer annealed under the denatured conditions. (C) Densitometry analysis of the gel shown in panel B. Tdp1 activity was calculated as the percentage of 14Y converted to 14P as a function of the concentration of furamidine. The horizontal line corresponds to 50% inhibition of Tdp1 activity.