Abstract
MicroRNAs (miRNAs) are incorporated into miRNP complexes and regulate protein expression post-transcriptionally through binding to 3′-untranslated regions of target mRNAs. Here we describe a recapitulation of let-7 miRNA-mediated translational repression in a cell-free system, which was established with extracts prepared from HEK293F cells overexpressing miRNA pathway components. In this system, both the cap and poly(A) tail are required for the translational repression, and let-7 directs the deadenylation of target mRNAs. Our work suggests that let-7 miRNPs containing Argonaute and GW182 impair the synergistic enhancement of translation by the 5′-cap and 3′-poly(A) tail, resulting in translational repression.
Keywords: let-7, miRNA, cell-free, recapitulation, deadenylation, translational repression
MicroRNAs (miRNAs) are ∼22-nucleotide (nt) small RNAs that regulate gene expression at the post-transcriptional level (Ambros 2004; Bartel 2004). There is growing evidence for the involvement of miRNAs in the regulation of genes that control cell growth and differentiation, as well as viral pathogenesis (Jopling et al. 2005; Sarnow et al. 2006). In animal cells, ∼70-nt precursor miRNAs (pre-miRNAs) are processed into ∼22-nt miRNAs by the Dicer–TRBP complex (Chendrimada et al. 2005; Gregory et al. 2005), and the miRNAs are incorporated into RNA–protein complexes, referred to as miRNPs. The miRNP binds to an mRNA with partial sequence homology with the miRNA and leads to its translational repression. Argonautes are key components of the miRNPs and interact with several proteins, including Dicer. Mammalian cells contain four Argonautes, Ago1 through Ago4, and all of these Ago proteins appear to associate with miRNAs and to function in translational repression (Meister et al. 2004; Pillai et al. 2004).
Several mechanisms of miRNA-mediated translational repression have been proposed, based on experiments using different systems (Jackson and Standart 2007; Pillai et al. 2007). They include repression at the initiation stage (Humphreys et al. 2005; Pillai et al. 2005), repression at post-initiation steps (Maroney et al. 2006; Nottrott et al. 2006; Petersen et al. 2006), and rapid degradation of target mRNAs (Rehwinkel et al. 2005; Behm-Ansmant et al. 2006; Wu et al. 2006). A crucial role of discrete cytoplasmic foci, the so-called P bodies (also known as GW bodies), in miRNA-mediated translational repression has been suggested (Eulalio et al. 2007; Jackson and Standart 2007; Parker and Sheth 2007; Pillai et al. 2007). P bodies contain factors involved in translational repression and mRNA decay. GW182 is an important P-body component, and is required for the deadenylation and accelerated decay of miRNA targets in Drosophila (Rehwinkel et al. 2005; Behm-Ansmant et al. 2006). In mammals, the silencing of GW182 expression impairs miRNA-mediated translational repression (Jakymiw et al. 2005; Liu et al. 2005).
To clarify how miRNAs repress translation, we established a mammalian cell-free system by employing let-7 miRNA as a model. Our data suggest that let-7 miRNPs direct the deadenylation of target mRNAs and impair the synergistic enhancement of translation by the 5′-cap and 3′-poly(A) tail, which results in translational repression.
Results and Discussion
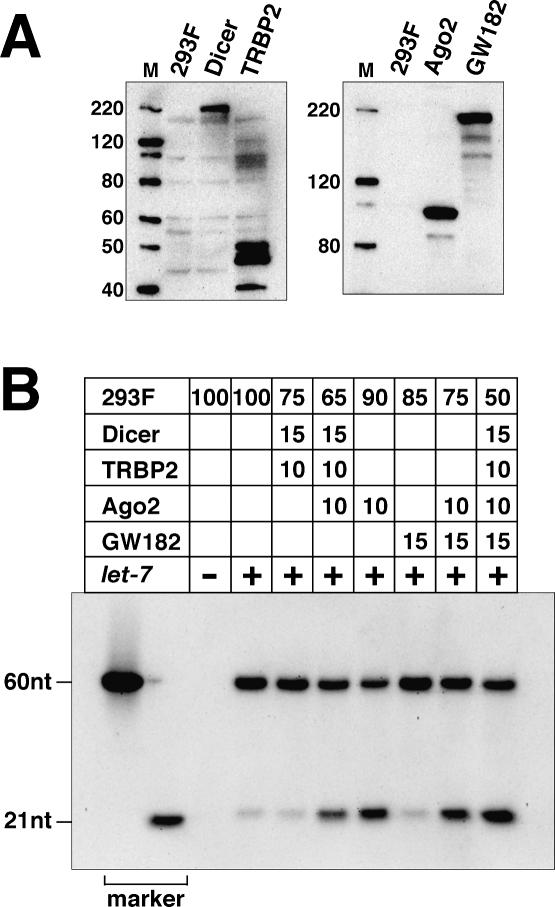
We transfected HEK293F cells (FreeStyle293F cells, Invitrogen) with expression vectors encoding Flag-tagged Dicer, TRBP2, Argonaute2 (Ago2), and GW182, and prepared extracts from each of the cell lines (Fig. 1A). We then mixed these cell extracts at various ratios and performed cell-free experiments. Since the endogenous expression of let-7 in 293F cells is very low, we could monitor the effect of the chemically synthesized let-7 miRNA introduced in the cell-free system.
Figure 1.
Overexpression of Ago2 stimulates the processing of let-7 pre-miRNA. (A) Expression of Dicer, TRBP2, Ago2, and GW182 in HEK293F cells. The expressed proteins were detected by Western blotting using an anti-Flag M2 antibody (Sigma). The sizes (in kilodaltons) of the molecular markers (see lane M) are indicated on the left. (B) Processing assay of let-7 pre-miRNA. Chemically synthesized let-7 pre-miRNA was incubated with extracts prepared from 293F cells, or the mixed extracts composed of the extracts prepared from 293F cells overexpressing Dicer, TRBP2, Ago2, or GW182, at the ratios indicated above the figure. The RNA was then extracted, and let-7 was detected by Northern blotting. Markers are a 60-nt (60nt) let-7 pre-miRNA and a 21-nt (21nt) let-7 siRNA. Five-hundred nanograms of RNA were loaded in each lane, except for the markers.
While we were developing the cell-free system that recapitulates miRNA-dependent translational repression, we noticed that external pre-miRNAs are poorly processed into their mature forms in 293F cell extracts (Fig. 1B). The overexpression of Dicer and TRBP2, which are the core components of the miRNA processor, did not stimulate the conversion of the preform to the mature form, as shown in Figure 1B. However, the addition of Ago2 (or Ago1) (data not shown) remarkably improved the processing efficiency. Moreover, the overexpression of Ago2 alone was sufficient to enhance the pre-miRNA processing. GW182, which interacts with the Argonaute proteins, did not further stimulate the processing activity (Fig. 1B).
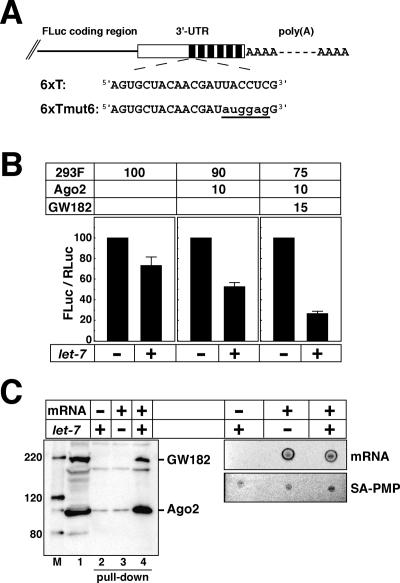
To assay the effects of let-7, we constructed firefly luciferase (FLuc) reporter mRNAs containing six let-7 target sequences (6xT), derived from the Drosophila lin-41 ortholog (Pasquinelli et al. 2000), in the 3′-untranslated region (UTR) (Fig. 2A). As a control, we also made a 6xTmut6 construct, in which the seeding region of the let-7 target sequence, UACCUC, was mutated to AUGGAG (Fig. 2A, underlined).
Figure 2.
GW182 enhances let-7 miRNA-mediated translational repression. (A) Schematic representations of the firefly luciferase mRNAs FLuc-6xT and FLuc-6xTmut6. (B) Translation of capped and polyadenylated FLuc-6xT mRNA in the absence (−) or presence (+) of let-7. Cell extracts prepared from control 293F cells and 293F cells overexpressing Flag-Ago2 and Flag-GW182 (see Fig. 1A) were used in the combinations indicated above the figure. The FLuc and RLuc activities were measured, and the FLuc-to-RLuc activity ratio in the reaction without let-7 was set at 100. The data shown constitute an average of at least three independent experiments, with standard deviations. (C) Biotinylated FLuc-6xT mRNAs (capped and polyadenylated) were incubated with the mixed extract (Ago2 + GW182; see lane 1) in the absence (−) or presence (+) of let-7. The mRNAs were captured with SA-PMPs. (Left panel) The proteins bound to the mRNAs were detected using an anti-Flag M2 antibody (lane 1, Ago2 + GW182 extract; lanes 2–4, pull-down). The sizes (in kilodaltons) of the molecular markers (M) are indicated on the left. (Right panel) The mRNAs captured with SA-PMPs were detected by dot hybridization (see the text).
The translation of capped and polyadenylated FLuc-6xT mRNA was examined in the absence (−) or presence (+) of let-7. Simultaneously, capped and nonadenylated Renilla luciferase (RLuc) mRNA was translated to normalize the FLuc activities in different experiments. In the 293F cell extracts, the let-7-mediated translational repression was ∼30%, while it was ∼50% with the extracts overexpressing Ago2 (Fig. 2B). The repression was significantly enhanced, to >70%, by the addition of an extract from cells overexpressing GW182 (Fig. 2B). These data indicate that GW182 is involved in miRNA-dependent translational repression in mammals and are consistent with previous reports (Jakymiw et al. 2005; Liu et al. 2005). In the following experiments, we used the mixed extract containing overexpressed Ago2 and GW182 (Ago2 + GW182). (Note: In some of the later experiments, we prepared different lots of extracts and used a mixture of extracts composed of 293F, Ago2, and GW182 at a ratio of 80:10:10.)
To examine the interactions of Ago2 and GW182 with the target mRNA, biotinylated FLuc-6xT mRNA (capped and polyadenylated) was prepared and incubated in the translation mixture containing the Ago2 + GW182 extracts (Fig. 2C, lane 1). Streptavidin paramagnetic particles (SA-PMPs, Promega) were used to capture the biotinylated mRNA–protein complexes. While the mRNAs were recovered from the extracts either in the absence or presence of let-7 (Fig. 2C, right panel), Flag-Ago2 and Flag-GW182 were specifically detected when let-7 was presented in the translation reaction mixture (Fig. 2C, left panel, cf. lanes 3 and 4). These data indicate that let-7 recruits the miRNP containing Ago2 and GW182 to the target mRNA in this cell-free system.
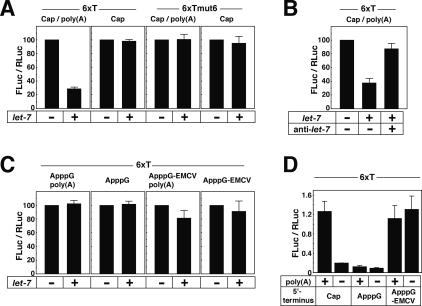
By using the Ago2 + GW182 extracts, we examined the translation of FLuc-6xT and FLuc-6xTmut6 mRNAs. Let-7 repressed the translation of capped and polyadenylated FLuc-6xT mRNA to <30%, but did not affect the translation of capped and polyadenylated FLuc-6xTmut6, which indicates that the repression is sequence-specific [Fig. 3A, Cap/poly(A)]. The capped and nonadenylated FLuc-6xT mRNA, as well as FLuc-6xTmut6, was not repressed by let-7, which strongly suggests that the translational repression is mediated through the poly(A) tail (Fig. 3A, Cap). The importance of the poly(A) tail in both in vivo and in vitro miRNA-mediated translational repression has been reported (Humphreys et al. 2005; Wang et al. 2006). The let-7-dependent translational repression was relieved by the addition of anti-let-7 RNA (Fig. 3B), which further supports the idea that the translational repression is sequence specific.
Figure 3.
The cap and poly(A) tail are important for the let-7-mediated translational repression. In the following experiments, Ago2 + GW182 extracts (see the text) were used. The FLuc and RLuc activities were measured, and the FLuc-to-RLuc activity ratio in the reaction without let-7 was set at 100, except in the experiment shown in D. The data shown constitute an average of at least three independent experiments, with standard deviations. (A) Capped FLuc-6xT and FLuc-6xTmut6 mRNAs with or without a poly(A) tail were translated in the absence (−) or presence (+) of let-7. (B) Capped and polyadenylated FLuc-6xT mRNA was translated in the absence (−) or presence (+) of let-7 and anti-let-7 (Ambion). (C) ApppG-FLuc-6xT and ApppG-capped EMC VIRES-FLuc-6xT mRNAs with or without a poly(A) tail were translated in the absence (−) or presence (+) of let-7. (D) Comparison of translation efficiencies of different constructs of FLuc-6xT reporter mRNAs in the absence of let-7. The average number of light units for the Cap/poly(A) mRNA was ∼7,000,000.
Next, we asked whether the let-7-mediated repression targets the function of the cap structure. We prepared FLuc-6xT mRNAs with a nonphysiological A(5′)ppp(5′)G cap (ApppG) and FLuc-6xT mRNAs with A(5′)ppp(5′)G-capped EMCV IRES (ApppG-EMCV). The A(5′)ppp(5′)G cap does not function in canonical translation initiation. The ApppG-FLuc-6xT mRNAs were not translationally repressed, irrespective of polyadenylation (Fig. 3C). On the other hand, the translation of the ApppG-EMCV IRES-FLuc-6xT mRNAs was slightly repressed by let-7, especially when they were polyadenylated (Fig. 3C). These data indicate that not only the poly(A) tail but also the cap structure is important for the let-7-mediated translational repression. This observation is consistent with the finding that miRNAs repress translation at the initiation step (Humphreys et al. 2005; Pillai et al. 2005).
A comparison of the translational efficiencies of the different FLuc-6xT constructs in the absence of let-7 is shown in Figure 3D. In this system, the 7-methyl-G cap (Cap) and poly(A) tail act synergistically to enhance translation. On the other hand, EMCV-IRES increased translation up to the same level as the Cap/poly(A) construct. In the translation experiments, we adjusted the concentration of mRNAs by weight. Since there is a difference in length by >10% between the polyadenylated and nonadenylated mRNAs, the molar concentration of the polyadenylated mRNAs in the translation reaction mixture should be less than that of the nonadenylated mRNAs. Therefore, we consider the polyadenylated and nonadenylated ApppG-EMCV mRNAs to have been comparably translated. The reason for the slight difference in the susceptibility to let-7 between the polyadenylated and nonadenylated ApppG-EMCV mRNAs is not immediately clear.
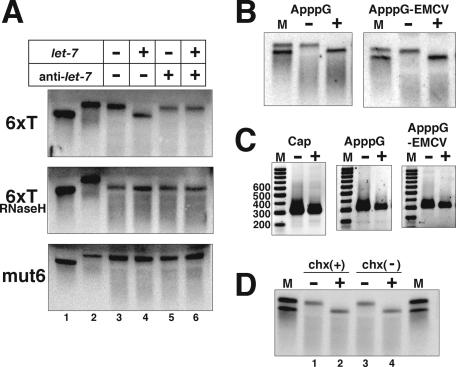
Analyses of the mRNAs by Northern blotting revealed that the capped and polyadenylated FLuc-6xT mRNAs, but not the capped and polyadenylated FLuc-6xTmut6 mRNAs, are shorter, nearly the same length as the nonadenylated mRNAs, after a 60-min incubation in the presence of let-7 (Fig. 4A, 6xT and mut6, cf. lanes 3 and 4). MiRNA-mediated mRNA deadenylation reportedly occurs in mammalian cells and zebrafish (Giraldez et al. 2006; Mishima et al. 2006; Wu et al. 2006). Therefore, we analyzed the poly(A) tail of the FLuc-6xT mRNAs by an RNaseH cleavage assay with oligo(dT). After the RNaseH treatment, the bands of the FLuc-6xT mRNAs shifted to almost the same position as the nonadenylated mRNAs under all conditions tested. The results indicated that the FLuc-6xT mRNAs are deadenylated in a let-7-dependent manner. The addition of anti-let-7 RNA prevented deadenylation (Fig. 4A, 6xT and mut6, cf. lanes 4 and 6). To address whether the deadenylation is the cause or the result of translational repression, we analyzed the poly(A) lengths of the ApppG-FLuc-6xT and ApppG-EMCV IRES-FLuc-6xT mRNAs. They were also deadenylated in the presence of let-7, as revealed by Northern blotting and PAT assays (Fig. 4B,C). Furthermore, let-7-dependent deadenylation still occurred in the presence of cycloheximide (chx), an inhibitor of translation, and thus it is independent of translation (Fig. 4D). These data indicate that the let-7-mediated deadenylation in this cell-free system is not the result of translational repression.
Figure 4.
Let-7 miRNAs direct deadenylation of the target mRNAs. (A) Analyses of capped and polyadenylated FLuc-6xT (6xT) and FLuc-6xTmut6 (mut6) mRNAs by Northern blotting. As molecular markers, nonadenylated and polyadenylated transcripts (capped) were loaded in lanes 1 and 2, respectively. After 60 min of translation in the absence (−) or presence (+) of let-7 and anti-let-7, the RNAs were extracted from the reaction mixture, and similar amounts were loaded in each lane, from lanes 3–6. In the middle panel, the extracted RNAs, as in the top panel (6xT), were annealed with oligo(dT) and subjected to RNaseH digestion. (B) Polyadenylated ApppG-FLuc-6xT and ApppG-capped EMCV IRES-FLuc-6xT mRNAs were translated in the absence (−) or presence (+) of let-7. In lane M, polyadenylated (longer) and nonadenylated (shorter) transcripts were loaded as markers. (C) PAT assay of polyadenylated FLuc-6xT mRNAs extracted after 60 min of translation in the absence (−) or presence (+) of let-7. The structures of the 5′ termini are indicated above the figures. (D) Capped and polyadenylated FLuc-6xT mRNAs were incubated in the translation reaction mixture for 60 min in the presence or absence of chx. Then, RNAs were extracted and FLuc-6xT mRNAs were detected by Northern blotting. The minus (−) and plus (+) signs above the lanes indicate the absence and presence of let-7. In lane M, polyadenylated (longer) and nonadenylated (shorter) transcripts were loaded as markers.
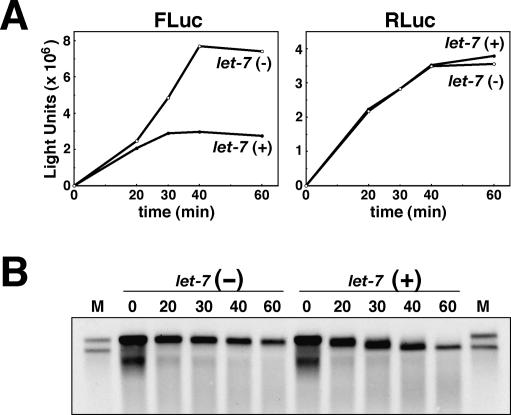
To further clarify the correlation between mRNA deadenylation and translational repression, we performed parallel time-course analyses of the luciferase expression and mRNA deadenylation of the 7-methyl-G-capped and polyadenylated FLuc-6xT mRNA (Fig. 5). In the presence of let-7, the synthesis of firefly luciferase (FLuc) completely stopped at the 30-min time point, while it continued to the 40-min time point in the absence of let-7 (Fig. 5A, left panel). The synthesis of Renilla luciferase (RLuc) did not stop until the 40-min time point in either the presence or absence of let-7 (Fig. 5A, right panel). On the other hand, the mRNA deadenylation had proceeded in the presence of let-7, but was not completed, by the 30-min time point (Fig. 5B). These data indicate the strong correlation between mRNA deadenylation and translational repression. However, since the mRNA still retained a short poly(A) tail when the translation stopped, it is possible that there is some direct effect of the let-7 miRNP on translation.
Figure 5.
Strong correlation between mRNA deadenylation and translational repression. (A) Time-course analysis of luciferase synthesis. Capped and polyadenylated FLuc-6xT mRNA and capped RLuc mRNA were translated. Aliquots were taken for luciferase assays at time points 0, 20, 30, 40, and 60 min from the start of translation. (B) In parallel with the analyses of luciferase activities, RNAs were extracted from the aliquots taken at each time point, and were subjected to Northern hybridization analyses using the FLuc probe.
The role of the poly(A) tail in translation has been revealed. The physiological 7-methyl-G cap and poly(A) tail function synergistically to regulate translational efficiency (Gallie 1991). The poly(A)-binding protein (PABP) interacts with the translation initiation factor eIF4G, a component of the cap-binding protein complex eIF4F (Gingras et al. 1999). PABP functions as a translational initiation factor and enhances the initiation complex formation (Kahvejian et al. 2005). Interference with the interaction of PABP and eIF4G results in the inhibition of poly(A)-dependent translation and Xenopus oocyte maturation (Wakiyama et al. 2000). Changes in the length of the poly(A) tail are critical for translational regulation in various organisms, especially at early stages of development. Cytoplasmic polyadenylation increases translation and mRNA stability, while deadenylation can cause translational repression and mRNA decay. These processes are controlled by proteins that bind to the 3′-UTR of target mRNAs (Wickens et al. 2002). Recently, Mishima et al. (2006) showed that miR-430 directs the deadenylation of nanos1 mRNA and represses translation during zebrafish embryogenesis. By using nonnatural ApppG-capped mRNA, they demonstrated that the deadenylation was a cause, but not a result, of translational repression. This is consistent with our work. In our cell-free system, let-7-dependent deadenylation does not require active translation and is independent of the structure of the mRNA 5′ terminus. These results strongly suggest that the deadenylation is a cause of translational repression.
In conclusion, we established a mammalian cell-free system that recapitulates pre-miRNA processing and miRNA-mediated translational repression. In this system, the cap and the poly(A) tail are both required for the translational repression. The results shown in this work suggest that let-7 miRNAs recruit miRNP complexes containing Argonaute and GW182 to let-7 target mRNAs, resulting in deadenylation, which in turn abolishes the cap–poly(A) synergy and represses translation. However, we cannot exclude the possibility that the let-7 miRNP has some direct effect on translation per se, in addition to the indirect effect of mRNA deadenylation. It could be possible that poly(A) is involved in recruiting miRNPs containing a factor that inhibits the function of the cap-binding protein, eIF4E. This mechanism could also explain the reason for the requirement of both the cap and poly(A) for translational repression. The system and the strategy presented in this paper will be powerful tools in studying the mechanisms of miRNA-mediated translational repression.
Materials and methods
RNA experiments
Chemically synthesized let-7 pre-miRNA was obtained from B-bridge International, and the let-7 small interfeing RNA (siRNA) was obtained from TaKaRa Bio. Anti-let-7 was obtained from Ambion (AM10048). The sequences of the let-7 pre-miRNA and the let-7 siRNA used in this work are as follows (the 5′ termini of both RNAs were phosphorylated): Let-7 pre-miRNA, 5′-UGAGGUAGUAGGUUGUAUAGUAGUAAUUACA CAUCAUACUAUACAAUGUGCUAGCUUUCU-3′ Let-7 siRNA, 5′-UGAGGUAGUAGGUUGUAUAGU-3′
Preparation of in vitro transcripts
The mRNAs encoding FLuc and RLuc were transcribed in vitro from the DNA templates described in the Supplemental Material. The 7-methyl-G(5′)ppp(5′)G-capped transcripts were prepared by using a mMESSAGE mMACHINE T7 kit (Ambion). The A(5′)ppp(5′)G-capped transcripts were prepared by using a MEGAscript T7 kit (Ambion) with the A(5′)ppp(5′)G cap analog (New England Biolabs). Poly(A) polymerase from yeast (USB Corporation) was used to add the poly(A) tail (>150 nt) to the transcripts. Biotinylated mRNA was prepared by using the mMESSAGE mMACHINE T7 kit with Bio-16-UTP (Ambion) at a final concentration of 1 mM. All of the transcripts were purified with a MEGAclear kit (Ambion) and were inspected by denaturing agarose gel electrophoresis.
RNaseH-poly(A) assay
The RNaseH-poly(A) assay was performed as described (Mishima et al. 2006) with a few modifications. Total RNAs were extracted from the translation reaction mixtures with a mirVana miRNA Isolation kit (Ambion), and 1 μg of total RNA was mixed with 50 pmol of oligo(dT)16. Samples were denatured for 5 min at 65°C, annealed for 10 min at 37°C, and then cooled in ice. Samples were mixed with 2.5 U of RNaseH (New England Biolabs) in 1× RNaseH buffer (50 mM Tris-HCl at pH 8.3, 75 mM KCl, 17 mM MgCl2, 10 mM DTT), and were incubated for 60 min at 37°C.
Northern blotting
RNAs were purified from reaction mixtures with the mirVana miRNA Isolation kit (Ambion). Northern blotting was performed as described previously (Wakiyama et al. 2005, 2006). To detect the let-7 miRNA, a 21-nt anti-let-7 oligo DNA, 5′-ACTATACAAC CTACTACCTCA-3′, was used.
PAT assay
The PAT assay was performed as described (Salles and Strickland 1999). Total RNA (100 ng) was reverse-transcribed using AMV reverse transcriptase (Invitrogen) with an oligo(dT)-anchor primer, 5′-GCGAGCTCCGCGGCCGCCTTTTTTTTTTTT-3′. By using aliquots of 1/10 to 1/100 dilutions as templates, PCR was performed with the primer 3′-UTR97seqF, 5′-ACATGACGATTATGAGGAGGTGCC-3′, which anneals to the 3′-UTR of the FLuc-6xT and FLuc-6xTmut6 mRNAs, and the primer PAT-anchor-R, 5′-AGCTGTTTCGCGAGCTC CGCGGCC-3′.
Cell culture and transfection
HEK293F cells (FreeStyle293F cells, Invitrogen) were grown in suspension using FreeStyle 293 Expression Medium (Invitrogen), as described by the manufacturer. The plasmids pCMV-SPORT/Flag-hDicer, pCMV-SPORT/Flag-TRBP2, pCMV-SPORT/Flag-Ago2, and pCMV-SPORT/Flag-GW182 were each transfected into FreeStyle293F cells at a ratio of 1 μg of DNA per 1 × 106 per milliliter of cells, by the use of 293 fectin (Invitrogen).
Cell-free experiments
Preparation of cell extracts
Cell extracts from 293F cells and 293F cells overexpressing Flag-tagged Dicer, TRBP2, Ago2, and GW182 were prepared based on the previously described method (Wakiyama et al. 2006). The cells were harvested 48 h after transfection by centrifugation at 1000g for 15 min at 4°C and were washed three times with ice-cold D-PBS (Invitrogen). The cells were then resuspended in lysis solution (40 mM Hepes-KOH at pH 7.4, 100 mM potassium acetate, 1 mM magnesium acetate, 1 mM dithiothreitol) at a cell density of ∼2 × 108 per milliliter, and were placed in a Cell Disruption Bomb (Parr Instrument Company). The cell suspension was subjected to 100 psi of nitrogen for 60 min with occasional agitation on ice. The homogenate produced upon pressure release was centrifuged at 1000g for 20 min at 4°C. The supernatant thus obtained was centrifuged at 10,000g for 10 min at 4°C. Creatine kinase (Roche Applied Science) was added at a concentration of 0.4 mg/mL extract, and the extract was divided into aliquots, frozen in liquid nitrogen, and stored at −80°C.
Let-7 pre-miRNA processing assay
Let-7 pre-miRNA was first heated for 3 min at 70°C, cooled to 25°C, incubated for 10 min at 25°C, and then cooled to 4°C. The let-7 pre-miRNA (3 pmol) was incubated with 30 μL of the cell extract, composed of the extracts prepared from 293F cells and 293F cells overexpressing Dicer, TRBP2, Ago2, and GW182 at the ratios indicated in Figure 1B, for 60 min at 37°C. The RNAs were then extracted and subjected to Northern blotting.
Translation experiments
The reactions were typically performed as follows: The cell extracts were preincubated with or without let-7 pre-miRNAs (0.1 pmol/μL) for 60 min at 37°C, as described in the section Let-7 Pre-miRNA Processing Assay, and then were cooled in ice. Translation reactions were then carried out in a final volume of 20–50 μL, containing a 60% volume of the preincubated cell extracts, 30 mM Hepes-KOH buffer (pH 7.4), 8 mM creatine phosphate, 0.5 mM spermidine, 1 mM ATP, 0.2 mM GTP, 20 μM amino acids, 1.5 mM magnesium acetate, 80 mM potassium acetate, 1 ng/μL of FLuc mRNA, and 1 (or 5) ng/μL RLuc mRNA. In the experiment shown in Figures 3B and 4A, 0.3 pmol/μL anti-let-7 was added when indicated in the figures. In the experiment shown in Figure 4D, 0.1 mg/mL chx was added when indicated in the figure. The luciferase activities were analyzed in a MiniLumat LB9506 (Berthold) with the Dual-Luciferase Reporter Assay System (Promega).
Biotin-RNA pull-down assay
The biotin-RNA pull-down assay was performed as described in the literature (Gerber et al. 2006), with some modifications. The cell extract (204 μL of Ago2 + GW182; see the text) was preincubated with or without let-7 pre-miRNAs (0.1 pmol/μL) for 60 min at 37°C and was cooled in ice. The biotinylated FLuc-6xT mRNAs (1 ng/μL, capped and polyadenylated) were incubated in 340 μL of translation reaction mixtures for 20 min at 37°C. SA-PMPs (Promega), pre-equilibrated in wash buffer (20 mM HEPES-KOH at pH 7.4, 150 mM NaCl, 1.5 mM MgCl2, 5% glycerol, 1.5 mM dithiothreitol, 0.2 mg/mL heparin, 0.2 mg/mL tRNA [Novagen], 0.25% BSA, protease inhibitor cocktail [Roche Diagnostics]), were added to the above reaction mixtures, and the mixtures were incubated for 30 min at 25°C. The SA-PMPs were then captured with a magnet, washed four times with 300 μL of the wash buffer, and boiled in 40 μL of SDS sample buffer. The supernatant was resolved on a 6% polyacrylamide/SDS gel and subjected to an immunoblot analysis using an anti-Flag M2 antibody (Sigma). The SA-PMPs were suspended in 10 μL of water, and 3 μL each of the suspension were spotted onto a nylon membrane. The FLuc-6xT mRNAs were detected by dot hybridization with the same probe as that used in the Northern hybridization.
Acknowledgments
We thank Ms. Tomoko Matsumoto and Ms. Yoko Kaitsu for excellent technical assistance. This work was supported by the RIKEN Structural Genomics/Proteomics Initiative (RSGI), and by the National Project on Protein Structural and Functional Analysis, Ministry of Education, Culture, Sports, Science and Technology of Japan.
Footnotes
Supplemental material is available at http://www.genesdev.org.
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.1566707
References
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Bartel D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Behm-Ansmant I., Rehwinkel J., Doerks T., Stark A., Bork P., Izaurralde E., Rehwinkel J., Doerks T., Stark A., Bork P., Izaurralde E., Doerks T., Stark A., Bork P., Izaurralde E., Stark A., Bork P., Izaurralde E., Bork P., Izaurralde E., Izaurralde E. mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Genes & Dev. 2006;20:1885–1898. doi: 10.1101/gad.1424106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chendrimada T.P., Gregory R.I., Kumaraswamy E., Norman J., Cooch N., Nishikura K., Shiekhattar R., Gregory R.I., Kumaraswamy E., Norman J., Cooch N., Nishikura K., Shiekhattar R., Kumaraswamy E., Norman J., Cooch N., Nishikura K., Shiekhattar R., Norman J., Cooch N., Nishikura K., Shiekhattar R., Cooch N., Nishikura K., Shiekhattar R., Nishikura K., Shiekhattar R., Shiekhattar R. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature. 2005;436:740–744. doi: 10.1038/nature03868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulalio A., Behm-Ansmant I., Izaurralde E., Behm-Ansmant I., Izaurralde E., Izaurralde E. P bodies: At the crossroads of post-transcriptional pathways. Nat. Rev. Mol. Cell Biol. 2007;8:9–22. doi: 10.1038/nrm2080. [DOI] [PubMed] [Google Scholar]
- Gallie D.R. The cap and poly(A) tail function synergistically to regulate mRNA translational efficiency. Genes & Dev. 1991;5:2108–2116. doi: 10.1101/gad.5.11.2108. [DOI] [PubMed] [Google Scholar]
- Gerber A.P., Luschnig S., Krasnow M.A., Brown P.O., Herschlag D., Luschnig S., Krasnow M.A., Brown P.O., Herschlag D., Krasnow M.A., Brown P.O., Herschlag D., Brown P.O., Herschlag D., Herschlag D. Genome-wide identification of mRNAs associated with the translational regulator PUMILIO in Drosophila melanogaster. Proc. Natl. Acad. Sci. 2006;103:4487–4492. doi: 10.1073/pnas.0509260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras A.C., Raught B., Sonenberg N., Raught B., Sonenberg N., Sonenberg N. eIF4 initiation factors: Effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem. 1999;68:913–963. doi: 10.1146/annurev.biochem.68.1.913. [DOI] [PubMed] [Google Scholar]
- Giraldez A.J., Mishima Y., Rihel J., Grocock R.J., Van Dongen S., Inoue K., Enright A.J., Schier A.F., Mishima Y., Rihel J., Grocock R.J., Van Dongen S., Inoue K., Enright A.J., Schier A.F., Rihel J., Grocock R.J., Van Dongen S., Inoue K., Enright A.J., Schier A.F., Grocock R.J., Van Dongen S., Inoue K., Enright A.J., Schier A.F., Van Dongen S., Inoue K., Enright A.J., Schier A.F., Inoue K., Enright A.J., Schier A.F., Enright A.J., Schier A.F., Schier A.F. Zebrafish MiR-430 promotes deadenylation and clearance of maternal mRNAs. Science. 2006;312:75–79. doi: 10.1126/science.1122689. [DOI] [PubMed] [Google Scholar]
- Gregory R.I., Chendrimada T.P., Cooch N., Shiekhattar R., Chendrimada T.P., Cooch N., Shiekhattar R., Cooch N., Shiekhattar R., Shiekhattar R. Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell. 2005;123:631–640. doi: 10.1016/j.cell.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Humphreys D.T., Westman B.J., Martin D.I., Preiss T., Westman B.J., Martin D.I., Preiss T., Martin D.I., Preiss T., Preiss T. MicroRNAs control translation initiation by inhibiting eukaryotic initiation factor 4E/cap and poly(A) tail function. Proc. Natl. Acad. Sci. 2005;102:16961–16966. doi: 10.1073/pnas.0506482102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R.J., Standart N., Standart N. How do microRNAs regulate gene expression? Sci. STKE. 2007;2007:re1. doi: 10.1126/stke.3672007re1. [DOI] [PubMed] [Google Scholar]
- Jakymiw A., Lian S., Eystathioy T., Li S., Satoh M., Hamel J.C., Fritzler M.J., Chan E.K., Lian S., Eystathioy T., Li S., Satoh M., Hamel J.C., Fritzler M.J., Chan E.K., Eystathioy T., Li S., Satoh M., Hamel J.C., Fritzler M.J., Chan E.K., Li S., Satoh M., Hamel J.C., Fritzler M.J., Chan E.K., Satoh M., Hamel J.C., Fritzler M.J., Chan E.K., Hamel J.C., Fritzler M.J., Chan E.K., Fritzler M.J., Chan E.K., Chan E.K. Disruption of GW bodies impairs mammalian RNA interference. Nat. Cell Biol. 2005;7:1267–1274. doi: 10.1038/ncb1334. [DOI] [PubMed] [Google Scholar]
- Jopling C.L., Yi M., Lancaster A.M., Lemon S.M., Sarnow P., Yi M., Lancaster A.M., Lemon S.M., Sarnow P., Lancaster A.M., Lemon S.M., Sarnow P., Lemon S.M., Sarnow P., Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific microRNA. Science. 2005;309:1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- Kahvejian A., Svitkin Y.V., Sukarieh R., M’Boutchou M.N., Sonenberg N., Svitkin Y.V., Sukarieh R., M’Boutchou M.N., Sonenberg N., Sukarieh R., M’Boutchou M.N., Sonenberg N., M’Boutchou M.N., Sonenberg N., Sonenberg N. Mammalian poly(A)-binding protein is a eukaryotic translation initiation factor, which acts via multiple mechanisms. Genes & Dev. 2005;19:104–113. doi: 10.1101/gad.1262905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Rivas F.V., Wohlschlegel J., Yates J.R., Parker R., Hannon G.J., Rivas F.V., Wohlschlegel J., Yates J.R., Parker R., Hannon G.J., Wohlschlegel J., Yates J.R., Parker R., Hannon G.J., Yates J.R., Parker R., Hannon G.J., Parker R., Hannon G.J., Hannon G.J. A role for the P-body component GW182 in microRNA function. Nat. Cell Biol. 2005;7:1261–1266. doi: 10.1038/ncb1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroney P.A., Yu Y., Fisher J., Nilsen T.W., Yu Y., Fisher J., Nilsen T.W., Fisher J., Nilsen T.W., Nilsen T.W. Evidence that microRNAs are associated with translating messenger RNAs in human cells. Nat. Struct. Mol. Biol. 2006;13:1102–1107. doi: 10.1038/nsmb1174. [DOI] [PubMed] [Google Scholar]
- Meister G., Landthaler M., Patkaniowska A., Dorsett Y., Teng G., Tuschl T., Landthaler M., Patkaniowska A., Dorsett Y., Teng G., Tuschl T., Patkaniowska A., Dorsett Y., Teng G., Tuschl T., Dorsett Y., Teng G., Tuschl T., Teng G., Tuschl T., Tuschl T. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol. Cell. 2004;15:185–197. doi: 10.1016/j.molcel.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Mishima Y., Giraldez A.J., Takeda Y., Fujiwara T., Sakamoto H., Schier A.F., Inoue K., Giraldez A.J., Takeda Y., Fujiwara T., Sakamoto H., Schier A.F., Inoue K., Takeda Y., Fujiwara T., Sakamoto H., Schier A.F., Inoue K., Fujiwara T., Sakamoto H., Schier A.F., Inoue K., Sakamoto H., Schier A.F., Inoue K., Schier A.F., Inoue K., Inoue K. Differential regulation of germline mRNAs in soma and germ cells by zebrafish miR-430. Curr. Biol. 2006;16:2135–2142. doi: 10.1016/j.cub.2006.08.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottrott S., Simard M.J., Richter J.D., Simard M.J., Richter J.D., Richter J.D. Human let-7a miRNA blocks protein production on actively translating polyribosomes. Nat. Struct. Mol. Biol. 2006;13:1108–1114. doi: 10.1038/nsmb1173. [DOI] [PubMed] [Google Scholar]
- Parker R., Sheth U., Sheth U. P bodies and the control of mRNA translation and degradation. Mol. Cell. 2007;25:635–646. doi: 10.1016/j.molcel.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Pasquinelli A.E., Reinhart B.J., Slack F., Martindale M.Q., Kuroda M.I., Maller B., Hayward D.C., Ball E.E., Degnan B., Muller P., Reinhart B.J., Slack F., Martindale M.Q., Kuroda M.I., Maller B., Hayward D.C., Ball E.E., Degnan B., Muller P., Slack F., Martindale M.Q., Kuroda M.I., Maller B., Hayward D.C., Ball E.E., Degnan B., Muller P., Martindale M.Q., Kuroda M.I., Maller B., Hayward D.C., Ball E.E., Degnan B., Muller P., Kuroda M.I., Maller B., Hayward D.C., Ball E.E., Degnan B., Muller P., Maller B., Hayward D.C., Ball E.E., Degnan B., Muller P., Hayward D.C., Ball E.E., Degnan B., Muller P., Ball E.E., Degnan B., Muller P., Degnan B., Muller P., Muller P., et al. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature. 2000;408:86–89. doi: 10.1038/35040556. [DOI] [PubMed] [Google Scholar]
- Petersen C.P., Bordeleau M.E., Pelletier J., Sharp P.A., Bordeleau M.E., Pelletier J., Sharp P.A., Pelletier J., Sharp P.A., Sharp P.A. Short RNAs repress translation after initiation in mammalian cells. Mol. Cell. 2006;21:533–542. doi: 10.1016/j.molcel.2006.01.031. [DOI] [PubMed] [Google Scholar]
- Pillai R.S., Artus C.G., Filipowicz W., Artus C.G., Filipowicz W., Filipowicz W. Tethering of human Ago proteins to mRNA mimics the miRNA-mediated repression of protein synthesis. RNA. 2004;10:1518–1525. doi: 10.1261/rna.7131604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai R.S., Bhattacharyya S.N., Artus C.G., Zoller T., Cougot N., Basyuk E., Bertrand E., Filipowicz W., Bhattacharyya S.N., Artus C.G., Zoller T., Cougot N., Basyuk E., Bertrand E., Filipowicz W., Artus C.G., Zoller T., Cougot N., Basyuk E., Bertrand E., Filipowicz W., Zoller T., Cougot N., Basyuk E., Bertrand E., Filipowicz W., Cougot N., Basyuk E., Bertrand E., Filipowicz W., Basyuk E., Bertrand E., Filipowicz W., Bertrand E., Filipowicz W., Filipowicz W. Inhibition of translational initiation by Let-7 microRNA in human cells. Science. 2005;309:1573–1576. doi: 10.1126/science.1115079. [DOI] [PubMed] [Google Scholar]
- Pillai R.S., Bhattacharyya S.N., Filipowicz W., Bhattacharyya S.N., Filipowicz W., Filipowicz W. Repression of protein synthesis by miRNAs: How many mechanisms? Trends Cell Biol. 2007;17:118–126. doi: 10.1016/j.tcb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Rehwinkel J., Behm-Ansmant I., Gatfield D., Izaurralde E., Behm-Ansmant I., Gatfield D., Izaurralde E., Gatfield D., Izaurralde E., Izaurralde E. A crucial role for GW182 and the DCP1:DCP2 decapping complex in miRNA-mediated gene silencing. RNA. 2005;11:1640–1647. doi: 10.1261/rna.2191905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salles F.J., Strickland S., Strickland S. Analysis of poly(A) tail lengths by PCR: The PAT assay. Methods Mol. Biol. 1999;118:441–448. doi: 10.1385/1-59259-676-2:441. [DOI] [PubMed] [Google Scholar]
- Sarnow P., Jopling C.L., Norman K.L., Schutz S., Wehner K.A., Jopling C.L., Norman K.L., Schutz S., Wehner K.A., Norman K.L., Schutz S., Wehner K.A., Schutz S., Wehner K.A., Wehner K.A. MicroRNAs: Expression, avoidance and subversion by vertebrate viruses. Nat. Rev. Microbiol. 2006;4:651–659. doi: 10.1038/nrmicro1473. [DOI] [PubMed] [Google Scholar]
- Wakiyama M., Imataka H., Sonenberg N., Imataka H., Sonenberg N., Sonenberg N. Interaction of eIF4G with poly(A)-binding protein stimulates translation and is critical for Xenopus oocyte maturation. Curr. Biol. 2000;10:1147–1150. doi: 10.1016/s0960-9822(00)00701-6. [DOI] [PubMed] [Google Scholar]
- Wakiyama M., Matsumoto T., Yokoyama S., Matsumoto T., Yokoyama S., Yokoyama S. Drosophila U6 promoter-driven short hairpin RNAs effectively induce RNA interference in Schneider 2 cells. Biochem. Biophys. Res. Commun. 2005;331:1163–1170. doi: 10.1016/j.bbrc.2005.03.240. [DOI] [PubMed] [Google Scholar]
- Wakiyama M., Kaitsu Y., Yokoyama S., Kaitsu Y., Yokoyama S., Yokoyama S. Cell-free translation system from Drosophila S2 cells that recapitulates RNAi. Biochem. Biophys. Res. Commun. 2006;343:1067–1071. doi: 10.1016/j.bbrc.2006.03.076. [DOI] [PubMed] [Google Scholar]
- Wang B., Love T.M., Call M.E., Doench J.G., Novina C.D., Love T.M., Call M.E., Doench J.G., Novina C.D., Call M.E., Doench J.G., Novina C.D., Doench J.G., Novina C.D., Novina C.D. Recapitulation of short RNA-directed translational gene silencing in vitro. Mol. Cell. 2006;22:553–560. doi: 10.1016/j.molcel.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Wickens M., Bernstein D.S., Kimble J., Parker R., Bernstein D.S., Kimble J., Parker R., Kimble J., Parker R., Parker R. A PUF family portrait: 3′UTR regulation as a way of life. Trends Genet. 2002;18:150–157. doi: 10.1016/s0168-9525(01)02616-6. [DOI] [PubMed] [Google Scholar]
- Wu L., Fan J., Belasco J.G., Fan J., Belasco J.G., Belasco J.G. MicroRNAs direct rapid deadenylation of mRNA. Proc. Natl. Acad. Sci. 2006;103:4034–4039. doi: 10.1073/pnas.0510928103. [DOI] [PMC free article] [PubMed] [Google Scholar]