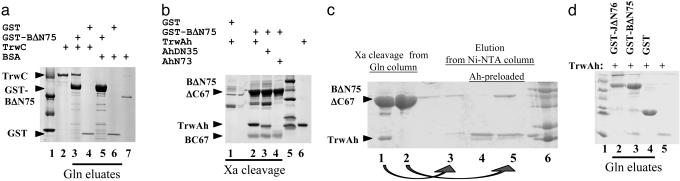
Fig. 1.
Protein-protein interactions detected by affinity chromatography. Soluble lysates containing GST fusion proteins were bound to glutathione-Sepharose resin, incubated with 20 μg of the indicated added proteins, and either eluted with glutathione (a and d) or cleaved with factor Xa protease (b and c). The figure shows 10% (a) or 12% (b-d) SDS/PAGE Coomassie-stained gels of eluted proteins. Molecular weight markers shown are 97.4 (not seen in b and c), 66, 45, 31, 21.5 (not seen in a), and 14.4 kDa (not seen in a). Lanes in a: 1, markers; 2, 2 μg of purified TrwC; 3-6, resin eluates; 3 and 5, lysates from pMTX501 (containing GST-TrwBΔN75); 4 and 6, lysates from pGEX-3X (containing GST); 3 and 4, bound proteins incubated with TrwC; 5 and 6, bound proteins incubated with BSA; 7, 1 μg of purified BSA. Lanes in b:1-4, Xa cleavage products; 1, lysates from pGEX-3X; 2-4, lysates from pMTX501; 1 and 2, incubation with TrwAh; 3, incubation with TrwAhΔN35; 4, incubation with TrwAhN73; 5, markers; 6, 2 μg of purified TrwAh. Arrowheads point to the two TrwB fragments obtained by factor Xa digestion. (c) TrwB-TrwA reciprocal interaction by affinity chromatography. Eluates from either glutathione-Sepharose resin by factor Xa digestion (lanes 1 and 2) or from Ni-NTA columns (lanes 3-5). Lanes: 1, TrwBΔN75 + TrwAh; 2, TrwBΔN75 + BSA; 3, eluate from lane 1 loaded on a Ni-NTA column; 4 and 5, Ni-NTA columns preloaded with TrwAh and then eluates from Xa digestions added (lane 4, from GST + TrwAh; lane 5, from TrwBΔN75 + BSA); 6, markers. Lanes in d: 1, markers; 2-4, eluates from lysates incubated with TrwAh; 2, pMTX609 (containing GST-TraJΔN76); 3, pMTX501; 4, pGEX-3X; 5, 1 μg of purified TrwAh.