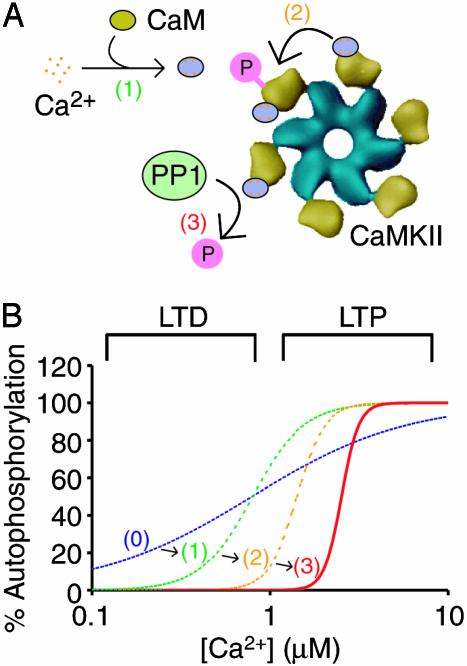
Fig. 5.
How a CaMKII-PP1 switch facilitates specificity in synaptic signaling. Shown are the molecular events causing the CaMKII-PP1 switch (A) and the effect of these events on the Ca2+ dependence of CaMKII autophosphorylation (B). Plotted in the graph is the percent CaMKII autophosphorylation versus the range of [Ca2+] in dendritic spines. If CaMKII activation were noncooperative, the activation curve would encompass the entire range of physiological Ca2+ values [dashed blue line and (0)]. Ultrasensitivity in CaMKII activation is imposed by both the cooperative binding of Ca2+ to CaM [dashed green line and (1)] and the dual requirement of CaM for autophosphorylation [dashed orange line and (2)]. Competitive dephosphorylation by PP1 further enhances the switch-like behavior and increases the Ca1/22+ value to the physiological [Ca2+] corresponding to LTP [solid red line and (3)]. The Ca2+ activation regions corresponding to LTP and LTD were determined based on the Ca2+ level in dendritic spines under depolarizing and resting potentials, respectively (3).